Minimally invasive resection of thymoma
Introduction
Thirty percent of all patients with thymoma are asymptomatic, whereas 40% show local symptoms related to the invasion of intrathoracic organs, and approximately 30% have generalized symptoms (1). Superior vena cava syndrome and weight loss may occur very rarely, and they are generally associated with aggressive tumors. Very rarely, patients may present with a fever or night sweats, which are more typical of lymphoma. Thymomas are frequently associated with “parathymic syndromes” (2,3). If these syndromes are present with an anterior mediastinal mass, the diagnosis is almost always a thymoma. The most commonly associated condition is myasthenia gravis (MG), which occurs in approximately 45% of patients with thymoma (1).
Although video-assisted thoracoscopic surgery (VATS) for thymectomy has become a popular approach over the last two decades, most cases are still performed through sternotomy. However, 14 different surgical techniques have been described for thymectomy (4). Although these techniques mostly report the type of entry into the mediastinum, surgical techniques inside the mediastinum rarely differ.
The types of incisions, the surgical techniques of exposure, visualization, and proper selection of equipment enable surgery to be performed via transcervical, extended transcervical, video-assisted thoracoscopy (VAT), and robotic approaches (e.g., right and/or left, right and cervical, left and cervical, subxiphoid and right and left, and cervical and subxiphoid approaches) (5).
A standard thymoma resection is a complete and en bloc resection of the thymoma and complete removal of the thymus, including the upper cervical poles and the surrounding mediastinal fat (6). Resection should also include all organs invaded by the thymoma, such as the pericardium, lungs, phrenic nerve, superior vena cava, aorta, and its main branches only under certain circumstances.
The author believes that the abovementioned requirements can be met by most minimally invasive techniques performed by experienced surgeons. For one surgeon, this could be a right-sided VATS Thymectomy, and for another, a left-sided robotic thymectomy or a transcervical intervention.
The aim of this article is to (I) question the radical oncological nature of minimally invasive techniques in patients with thymoma, (II) describe surgical techniques with VATS and robotics, and (III) present the results of minimally invasive thymoma resections.
Is a tissue diagnosis essential for the complete resection of an anterior mediastinal mass resection? Is minimally invasive resection an option?
The role of VATS thymectomy (VATT) as both a diagnostic tool and curative method has not been clearly established in contemporary practice (7). This depends on the nature of the disease, gender, age, and radiological findings of patients. Computed tomography has a 97% sensitivity in distinguishing a thymic mass from other anterior mediastinal masses (8), but the problem occurs when there is no associated MG (9). If pleural nodules are present and the mass is lobulated, it is probably a higher-stage thymoma, and minimally invasive resection is not an option (9). Computer tomography-guided needle aspiration biopsy is the most commonly used diagnostic modality if the lesion cannot be resected with minimally invasive techniques. However, many times, this may be insufficient to separate a mediastinal lymphoma from a thymoma if the pathologists are not experts. For instance, reports have shown that the diagnostic accuracy of a fine-needle aspiration biopsy may be as low as 77% (10). Possible complications of computer-guided aspiration biopsies, such as tumor seeding and massive hemothorax, have also been reported (11,12). Core or surgical biopsies are more reliable techniques than fine-needle aspiration biopsies. Mediastinotomy/mediastinoscopy or VATS biopsies could be recommended if the patient is a candidate for neoadjuvant treatment and radiological findings are difficult to differentiate a thymoma from a lymphoma. Video-assisted thoracoscopic resection of the suspicious anterior mediastinal mass for diagnostic purposes may be performed as the first-line approach when the lesion is small, encapsulated, and shows no invasion to the lungs, phrenic nerves, and major vessels (13), even if the patient does not have MG. It is recommended that an experienced VATS thymectomy surgeon perform this procedure after discussing the case in a multidisciplinary meeting.
In our department, for the last 15 years, we have suggested direct mediastinal mass resection without tissue diagnosis if the following are provided:
- A VATS resection candidate without invasion to the lungs and innominate vein.
- No B symptoms.
- A patient with MG.
Who are VATS resection candidates?
A recently published systematic review showed that the main concerns regarding VATS resection candidates are the size of the thymoma and Masaoka stage (14). This article demonstrated that the patients who were generally operated by VATS had tumor diameters <5 cm (15-18). Furthermore, in most studies, a clear radiological separation from the innominate vein and other vital organs, including the great vessels, heart, and trachea, with no evidence of local invasion, is a requirement (15-17). Size is predictable from computed chest tomography (CT) and is also used to predict the Masaoka stage. This may be difficult in patients with MG when CT is performed without intravenous contrast because of the possible side effects of contrast. In our experience, open conversions due to great vessel invasion could be the result of performing CT without contrast enhancement. Contrast-enhanced CT in patients without MG and magnetic resonance imaging in patients with MG could be the radiological modality choice when vessel invasion is suspected. Cheng and colleagues were unable to differentiate stage I from stage II thymomas despite rigid evaluation (19). Based on their experience, VATS candidates can be selected using the following criteria: anterior mediastinal mass, a distinct fat plane between the tumor and vessels, unilateral tumor predominance, encapsulation of the tumor, no mass compression effect, no pericardial and pleural effusions, and no hemi-diaphragmatic paralysis and encasement of the great vessels (19).
Based on our experience, VAT may be the last step for evaluating a possible candidate (20).
If the planned VATS resection is compromised, the surgeon should quickly consider open conversion. Open conversion is not a complication if the planned resection is compromised (6). Before any dissection, the thymoma should be located and possible minimally invasive resection should be assessed in terms of potential major vascular (left innominate vein as minimal), phrenic nerve, and sternum invasion (6).
The theoretical risk of incomplete resection or capsular disruption may cause earlier recurrence and decrease survival. This possibility has led surgeons to avoid minimally invasive thymoma resection for several years. The primary aim of the surgeon should always be a complete resection. In our study, we developed standard terms, policies, and definitions for minimally invasive thymoma resection and defined the general principles of minimally invasive resections, incisions, exposure and extent of resections, as well as open conversion criteria. We also generated ideas regarding performing operations and handling specimens (6).
Thymomectomy or thymothymectomy
Although there are academic reports that promote only thymic mass resection without resecting the thymus or mediastinal fat, most of the authors and international societies have recommended en bloc resection of the thymic mass with the thymus and mediastinal tissue.
A recent study from Shangai evaluated the surgical outcomes of tumor resection with or without total thymectomy for thymic epithelial tumors using the Chinese Alliance for Research in Thymomas retrospective database (21). Patients without preoperative therapy, who underwent surgery for early-stage (Masaoka-Koga stages I and II) tumors, were divided into thymectomy and thymomectomy groups according to the resection extent of the thymus. Seven hundred and ninety-six patients in the thymectomy group and 251 patients in the thymomectomy group were compared. The improvement rate of MG was higher after thymectomy than after thymomectomy (91.6% vs. 50.0%, P<0.001). Ten-year overall survival was similar between the two groups (90.9% after thymectomy and 89.4% after thymomectomy). The recurrence rate was 3.1% after thymectomy and 5.4% after thymomectomy, with no significant difference between the two groups. However, in patients with Masaoka-Koga stage II tumors, recurrence was significantly less in the thymectomy group than in the thymomectomy group (2.9% vs. 14.5%, P=0.001).
We believe that thymothymectomy, instead of tumor resection alone, should still be the choice of surgical standard for thymic malignancies, particularly in patients with concomitant MG.
Adjuvant radiotherapy and sternum-sparing technique
In the past, one of the major postoperative problems was sternal healing after high-dose radiotherapy, particularly in those who were prescribed high-dose corticosteroids or immunosuppressive treatment for MG. These patients with immunosuppressive treatment and corticosteroids may benefit from either VATS resection or anterior thoracotomy to preserve the sternum. If open conversion is required during a VATS thymoma resection, we suggest mini-thoracotomy instead of sternotomy to prevent possible sternal infection or dehiscence problems.
Frequently used minimally invasive surgical techniques and common intraoperative events
In the literature, differences in both surgical approaches and treatment strategies for thymoma are apparent. Many surgeons seem to prefer a complete thymectomy VATS approach. Extended thymectomy with VATS is generally being performed by experienced VATS surgeons. Although abovementioned is a general sense, a recent review does not support the authors’ thoughts. It has been shown that a higher proportion of extended thymectomy is performed by open surgeons (98.5%; range, 63.6–100%) than by VATS surgeons (79.9%; range, 40.8–100%) (14). This means that most of the open surgeons prefer an extended thymectomy technique, whereas VATS surgeons mostly prefer a complete thymectomy operation. We believe that VATS surgeons are striving to develop their techniques. Regarding more extensive thymectomy operations through subxiphoid or bilateral approaches, authors also demonstrated that a greater percentage of VATS surgeons perform a “partial” thymectomy (20.8%; range, 0–59.2%) than that of open surgeons (1.5%; range, 0–36.4%) (14). Many surgeons prefer the unilateral VATS approach with most of thymectomy operations being performed through the right side. Many surgeons indicate that the cavity is larger on the right and use both the superior vena cava and phrenic nerve as landmarks, whereas others prefer to perform the operations on the side of the tumor (18,22-24). Some surgeons, including us, preferred the right-sided approach under all circumstances except in cases of obvious left-sided thymoma (25,26). Maniscalco et al. reported that their VATS approach was mostly left-sided (27). In addition, other surgeons, including us, prefer bilateral VATS approaches for larger tumors (16,17,22-24,28). Tagawa as well as Zielinski used a cervico-xiphoidal-thoracic approach (17,29). A recent experience with robot assisted thoracic surgery (RATS) showed that 20 patients with large tumors (with a median size of 6 cm) were mostly approached through the right side. Combined resection of adjacent structures, including the pericardium, lung, and phrenic nerve, was frequently performed in this subset of patients. Patients who underwent RAT, compared with those who underwent an open approach, had lower blood loss (25 vs. 150 mL), were more frequently managed with a single chest tube (85% vs. 56%), and had a shorter median length of stay (3 vs. 4 days). Unfortunately, three patients undergoing RAT (15%) underwent open conversion (30). In addition, Rückert et al. favored the left-sided robotic approach (31). A European multi-institutional series, which also excluded Berlin’s experience (32) collected 134 patients with a clinical diagnosis of thymoma were operated on using a left-sided (38%), right-sided (59.8%), or bilateral (2.2%) robotic approach. Mostly right-sided operations were performed. Twelve (8.9%) patients needed open conversion (32). Neither vascular and nerve injuries nor perioperative mortality occurred. A total of 23 (17.1%) patients experienced postoperative complications (32). Median hospital stay was 4 days (range, 2–35 days) (32). Mean diameter of resected tumors was 4.4 cm (range, 1–10 cm), Masaoka stage was I in 46 (34.4%) patients, II in 71 (52.9%), III in 11 (8.3%), and IVa/b in 6 (4.4%) cases. Minimally invasive mediastinal surgery has potential threats of major catastrophes. We recently published the catastrophes that occurred during VATS thymectomy operations (33). In our experience, which comprises 441 VAT thymectomy/thymothymectomy operations, catastrophic complications were identified in seven (1.5%) patients. Three major innominate vein injuries, one superior vena cava injury, one aortic injury, one sudden cardiac arrest, and one diaphragmatic injury were reported. The first catastrophe for the experienced surgeon was his 96th case. Only two (28.6%) of the patients had thymoma, and the mean body mass index was 23.9±2.8. A mean of 1.7±1.4 (minimum: 0, maximum: 3) units of blood were transfused; a postoperative intensive care unit stay of 20.6±25.7 h and a hospital stay of 8.4±7.9 days were presented (33). We did not report any mortality (33).
Should lymph node resection be routine at the time of thymoma resection with minimally invasive surgery?
In the last decade, the rate of lymph node metastases was shown to be 1.8% in thymic epithelial tumors (34,35). Until recently, Kondo and Monden’s paper was a unique publication because their results were supported by a large database demonstrating both the incidence and prognostic significance of nodal metastases in patients with thymoma (36). In this study, 2,227 patients with thymoma were evaluated. Of the 2,227 patients, 442 underwent lymph node dissection. Although the median number of resected nodes was very low (2), 59 patients (13.3%) were shown to have lymph node invasion; these were patients with small thymomas. In conclusion, nodal metastases may occur more frequently than identified in the last decade. Thus, anterior mediastinal lymph node resection should be performed in routine investigations for thymoma. One of the most important conclusions of this paper is that young patients with small thymomas are the ones who may have a possibility to have metastatic lymph nodes, and typically these patients are suitable candidates for minimally invasive thymoma resection.
The surgical technical suggestions for lymph node evaluation in minimally invasive thymoma resections have yet to be defined. The author performs anterior mediastinal lymph node resection routinely in minimally invasive thymoma resections and dissects the deeper region as part of the routine procedure in open surgeries.
In 2013, Park et al. (37) recommended routine dissection of all mediastinal lymph nodes in patients with thymic resection. The International Thymic Malignancy Interest Group and International Association for the Study of Lung Cancer have created a new TNM staging classification for thymic epithelial tumors to emphasize the pathological lymph node status (38,39).
Oncological outcomes of minimally invasive surgery
In 2011, Agasthian demonstrated that VATS thymectomy can be performed for Masaoka stages III and IV thymomas <5 cm in size, with only one recurrence over a median follow-up of 4.9 years (40). This finding has also been demonstrated in four posterior series (15,16,41,42). The overall survivals after thymoma resection in the first, second, and fifth years were higher in minimally invasive operations than in open operations or similar in both: 98.1–100%, 96.8–100%, and 83.3–100% in VATS vs. 94.4–100%, 90.7–100%, and 79.1–98.0% in open surgery (14). Three studies assessed the superior recurrence-free survival at 10 years postoperatively: 88.9–100% in VATS vs. 79.5–92.8% in open thymectomy (22,28,41).
Benefits of robotic surgery
Seventy-nine patients who were operated on for early-stage thymoma with da Vinci technology RATS were evaluated. They included one patient who needed open conversion, one patient who required a standard thoracoscopy and five patients who required extra incisions. No perioperative mortality occurred (43). The median diameter of resected thymoma was 3 cm (range, 1–12 cm), and median hospital stay was 3 days (range, 2–15 days) (43). Thirty patients (38%) were in Masaoka stage I and 49 patients (62%) in stage II. Seventy-four patients were alive, and five had died (four patients from non-thymoma-related causes and one from a diffuse intrathoracic recurrence) at a median follow-up of 40 months, and the five-year survival rate was calculated to be 90% (43). Although oncologic outcomes appear acceptable, longer follow-up is needed. In another recent study, the short-term outcomes of 46 patients at Masaoka stage I without MG were analyzed (44). Twenty-five patients underwent unilateral VATS, and 21 underwent unilateral RATS thymectomy. The postoperative hospital stay was shorter (3.7 vs. 6.7 days; P<0.01), and the duration of postoperative pleural drainage was also shorter in the RATS group (1.1 vs. 3.6 days; P<0.01). No patient in the RATS group underwent open conversion, and only one was converted in the VATS group. Another conclusion of the study was that robotic surgery is more expensive than VATS (44).
A recently published manuscript (45) compared RATS, VATS, and median sternotomy (MS), the three approaches for extended thymectomy operation in the treatment of early-stage thymomas. Some of the perioperative parameters, such as the volume of intraoperative blood loss, mean duration of postoperative pleural drainage, volume of pleural drainage, and mean duration of hospital stay, showed significant differences. RATS causes a reduction in the postoperative drainage duration and volume (2.88 vs. 3.77 and 4.41 days, 352.2 vs. 613.9 and 980 mL) and hospital stay versus the MS and VATS groups (4.3 vs. 5.5 and 6.6 days) (45). In this study, no postoperative complications occurred in the RATS or VATS group, and only three patients experienced postoperative complications in the MS group. According to this study, RATS and VATS appear both feasible and safe for early-stage thymoma resection. RATS seems to be less invasive than VATS in terms of shorter duration of pleural drainage, less pleural drainage, and a shorter postoperative hospital stay (45).
It has been demonstrated that the robot-assisted thymectomy has the potential to perform a complete thymectomy. Identification of the contralateral phrenic nerve may be a problem when VATS is used. However, the phrenic nerve can be visualized easily when the optical capabilities of the da Vinci robot are used (Intuitive Surgical Inc., Sunnyvale, CA). Sometimes even with robotic use, the contralateral phrenic nerve cannot be visualized. Indocyanine green fluorescence imaging during RATS was used in a study with the aim of identifying the contralateral phrenic nerve (46). Indocyanine green did not cause any complications, and no injuries occurred to the phrenic nerve. In addition, the contralateral phrenic nerve could be visualized in 80% of patients during right-sided robotic thymectomy. Robotic technology was reported to have the potential to maximize thymic tissue resection (46).
Surgical techniques used in our clinical practice
Anesthesia
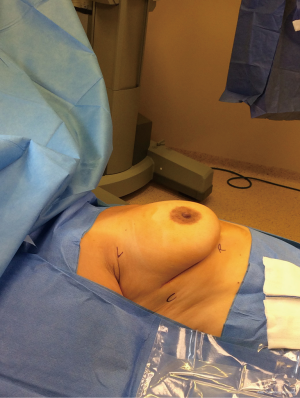
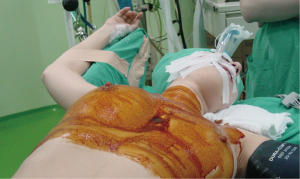
We always position the patient in a 30-degree semi-supine position. The patient is supported by a roll placed under the right shoulder, and the right arm is abducted over a support attached to a table when the procedure is going to be VATS (Figure 1). If the procedure is going to be a robotic one, the positioning is slightly different. Patients are intubated with a standard left, double-lumen tube and positioned supine. Arm placement is totally different from that in VATS since the arm is placed laterally and inferiorly (Figure 2). We almost always prefer the right-side approach for both VATS and robotic thymoma resections unless a thymoma is located completely on the left side near the phrenic nerve. The reason we prefer the right side is because it provides more room and has clear landmarks. We do not use carbon dioxide insufflation because we open the contralateral pleura for dissection of the left side, which may potentially cause a ventilation problem. Initially, 5 mL of bupivacaine (0.5% Marcaine; AstraZeneca, Istanbul) is injected into incisions as preemptive medication.
VATS thymothymectomy
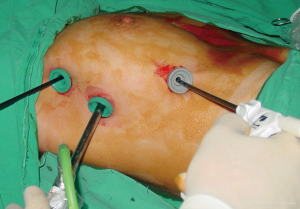
A 30-degree scope is used in VATS thymoma operations. The surgeon and camera man stand side-by-side, and the nurse stand on the other side (Figure 3). The hemithorax is evaluated for possible implants if the thymoma has invaded the mediastinal pleura. We also look for lymph nodes in the mediastinal fatty tissue. The right phrenic nerve is defined. The location of the thymoma and its relationships to both adjacent tissues and vessels are evaluated, such as pericardium involvement, innominate vein invasion, lung invasion, etc. We dissect the right-sided pericardiophrenic fatty tissue and open the left mediastinal pleura under the sternum and then resect all the mediastinal pleura on the left side. We do not touch the thymoma. Then, we begin the dissection of the thymus and remove it from the pericardium. After completion of the fatty tissue on the right and left inferior poles over the diaphragm, we elevate the thymus from the pericardium without touching the thymoma.
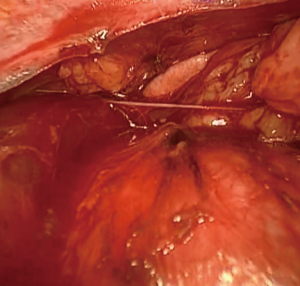
The dissection of the right-side mediastinal pleura parallel to the right phrenic nerve provides the visualization of both the superior vena cava and left innominate vein. This part of the operation is where accidents start to occur. Gentle traction on the superior poles provides a plane between the innominate vein and thymus. Starting from the right superior pole of the thymus and then continuing with the left pole under direct visualization provides visualization of the thymic vein or veins (Figures 4,5). By retracting the tissues and thymus toward the right side, the left half moves toward the ipsilateral side and dissection from the left phrenic nerve can be accomplished. Completion of the left thymus side dissection mobilizes the thymoma as the last part of the operation. The specimen is removed in an endobag. In principle, the tumor should be dissected as the last part of the operation, and surgery should be completed with the no-touch technique. The non-tumorous part of the thymus is dissected first, and these tissues are used for grasping and traction.

Robotic thymothymectomy
Three incisions are made around the mammary gland (Figure 6). A zero-degree scope is inserted from the middle port. After the docking is completed, intrathoracic exploration including the thymus is performed. Maryland bipolar forceps of the da Vinci are used to carefully dissect the fatty and thymic tissue off the pericardium because maintaining the integrity of the thymoma capsule is extremely important. The mediastinal pleura are opened from the diaphragm to the thoracic inlet under the sternum.
The right upper pole then the left upper pole is retracted using a moderate amount of tension as described in VATS. After retraction of the superior poles, the thymic vein is dissected and divided. Then, the thymoma is mobilized as the last step of the operation. The resected thymoma is removed with an endobag. A search for an anterior mediastinal lymph node is performed. If there is PET CT positivity in the paratracheal lymph nodes, a zero-down scope is placed and right-sided paratracheal nodes are removed. If the thymoma is large, there is a potential threat of innominate vein invasion. The console surgeon may contribute to the operation with the VATS technique for a while in order to staple the innominate vein and ensure that the resection produces negative margins (Figure 7).

Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors Nuria Novoa and Wentao Fang for the series “Minimally Invasive Thymectomy” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2018.04.02). The series “Minimally Invasive Thymectomy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Detterbeck FC, Parsons AM. Thymic tumors: a review of current diagnosis, classification, and treatment. Pearson’s Thoracic and esophageal surgery. 3rd edition. Philadelphia: Elsevier; 2008;131:1589-614.
- Souadjian JV, Enriquez P, Silverstein MN, et al. The spectrum of diseases associated with thymoma. Arch Intern Med 1974;134:374-79. [Crossref] [PubMed]
- Rosenow EC, Hurley BT. Disorders of the thymus. Arch Intern Med 1984;144:763-70. [Crossref] [PubMed]
- Rückert JC, Ismail M, Badakhshi H, Meisel A, Swierzy M. Thymectomy in myasthenia and/or thymoma. Zentralbl Chir 2014;139:121-32. [Crossref] [PubMed]
- Drachman DB. Myasthenia gravis. N Engl J Med 1994;330:1797-810. [Crossref] [PubMed]
- Toker A, Sonett J, Zielinski M, et al. Standard terms, definitions and policies for minimally invasive resection of thymoma. J Thorac Oncol 2011;6:S1739-42. [Crossref] [PubMed]
- Vaja R, Joshi V, Dawson AG, et al. Is a diagnostic video-assisted thoracoscopic thymectomy an acceptable first-line approach to the suspicious anterior mediastinal mass? J Minim Access Surg 2017;13:286-90. [Crossref] [PubMed]
- Yonemori K, Tsuta K, Tateishi U, et al. Diagnostic accuracy of CT guided percutanous cutting needle biopsy for thymic tumors. Clin Radiol 2006;61:771-5. [Crossref] [PubMed]
- Tseng YC, Hsieh CC, Huang HY, et al. Is Thymectomy Necessary in Nonmyasthenic Patients with Early Thymoma? J Thorac Oncol 2013;8:952-8. [Crossref] [PubMed]
- Morrissey B, Adams H, Gibbs AR, et al. Percutanous needle biopsy of the mediastinu: review of 94 procedures. Thorax 1993;48:632-7. [Crossref] [PubMed]
- Matsumoto K, Ashizawa K, Tagawa T, et al. Chest wall implantation of a thymic cancer after computed tomography-guided core needle biopsy. Eur J Cardiothorac Surg 2007;32:171-3. [Crossref] [PubMed]
- Yaacob Y, Muda S, Zakaria R. Fatal mediastinal biopsy. How interventional radiology saves the day. Ann Thorac Med 2012;7:107-9. [Crossref] [PubMed]
- Carter BW, Marom EM, Detterbeck FC. Approaching the patients with an anterior mediastinal mass: a guide for clinicians. J Thorac Oncol 2014;9:S102-9. [Crossref] [PubMed]
- Xie A, Tjahjono R, Phan K, et al. Video-assisted thoracoscopic surgery versus open thymectomy for thymoma: a systematic review. Ann Cardiothorac Surg 2015;4:495-508. [PubMed]
- Chung JW, Kim HR, Kim DK, et al. Long-term results of thoracoscopic thymectomy for thymoma without myasthenia gravis. J Int Med Res 2012;40:1973-81. [Crossref] [PubMed]
- Liu TJ, Lin MW, Hsieh MS, et al. Video-assisted thoracoscopic surgical thymectomy to treat early thymoma: a comparison with the conventional transsternal approach. Ann Surg Oncol 2014;21:322-8. [Crossref] [PubMed]
- Tagawa T, Yamasaki N, Tsuchiya T, et al. Thoracoscopic versus transsternal resection for early stage thymoma: long-term outcomes. Surg Today 2014;44:2275-80. [Crossref] [PubMed]
- Ye B, Tantai JC, Ge XX, et al. Surgical techniques for early-stage thymoma: video-assisted thoracoscopic thymectomy versus transsternal thymectomy. J Thorac Cardiovasc Surg 2014;147:1599-603. [Crossref] [PubMed]
- Cheng YJ, Hsu JS, Kao EL. Characteristics of thymoma successfully resected by videothoracoscopic surgery. Surg Today 2007;37:192-6. [Crossref] [PubMed]
- Toker A, Erus S, Ziyade S, et al. It is feasible to operate on pathological Masaoka stage I and II thymoma patients with video-assisted thoracoscopy: analysis of factors for a successful resection. Surg Endosc 2013;27:1555-60. [Crossref] [PubMed]
- Gu Z, Fu J, Shen Y, et al. Thymectomy versus tumor resection for early-stage thymic malignancies: a Chinese Alliance for Research in Thymomas retrospective database analysis. J Thorac Dis 2016;8:680-6. [Crossref] [PubMed]
- Chao YK, Liu YH, Hsieh MJ, et al. Long-term outcomes after thoracoscopic resection of stage I and II thymoma: a propensity-matched study. Ann Surg Oncol 2015;22:1371-6. [Crossref] [PubMed]
- Odaka M, Akiba T, Yabe M, et al. Unilateral thoracoscopic subtotal thymectomy for the treatment of stage I and II thymoma. Eur J Cardiothorac Surg 2010;37:824-6. [Crossref] [PubMed]
- Yuan ZY, Cheng GY, Sun KL, et al. Comparative study of video-assisted thoracic surgery versus open thymectomy for thymoma in one single center. J Thorac Dis 2014;6:726-33. [PubMed]
- He Z, Zhu Q, Wen W, et al. Surgical approaches for stage I and II thymoma-associated myasthenia gravis: feasibility of complete video-assisted thoracoscopic surgery (VATS) thymectomy in comparison with trans-sternal resection. J Biomed Res 2013;27:62-70. [PubMed]
- Cheng YJ. Videothoracoscopic resection of encapsulated thymic carcinoma: retrospective comparison of the results between thoracoscopy and open methods. Ann Surg Oncol 2008;15:2235-8. [Crossref] [PubMed]
- Maniscalco P, Tamburini N, Quarantotto F, et al. Longterm outcome for early stage thymoma: comparison between thoracoscopic and open approaches. Thorac Cardiovasc Surg 2015;63:201-5. [Crossref] [PubMed]
- Pennathur A, Qureshi I, Schuchert MJ, et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg 2011;141:694-701. [Crossref] [PubMed]
- Zielinski M, Kudzdal J, Nabialek T. Transcervical-subxiphoid VATS maximal thymectomy for myasthenia gravis. Multimed Man Cardiothorac Surg 2005;2005:mmcts.2004.000836.
- Kneuertz PJ, Kamel MK, Stiles BM, et al. Robotic Thymectomy Is Feasible for Large Thymomas: A Propensity-Matched Comparison. Ann Thorac Surg 2017;104:1673-8. [Crossref] [PubMed]
- Rückert JC, Swiery M, Ismail M. Comparison of robotic and non robotic thoracoscopic thymectomy: A cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. [Crossref] [PubMed]
- Marulli G, Maessen J, Melfi F, et al. Multi-institutional European experience of robotic thymectomy for thymoma. Ann Cardiothorac Surg 2016;5:18-25. [PubMed]
- Özkan B, Toker A. Catastrophes during video-assisted thoracoscopic thymus surgery for myasthenia gravis. Interact Cardiovasc Thorac Surg 2016;23:450-3. [Crossref] [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial: a clinical study of 1320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. [Crossref] [PubMed]
- Kondo K, Monden Y. Lymphogenous and hematogenous metastasis of thymic epithelial tumors. Ann Thorac Surg 2003;76:1859-64; discussion 1864-5.
- Weksler B, Pennathur A, Sullivan JL, et al. Resection of thymoma should include nodal sampling. J Thorac Cardiovasc Surg 2015;149:737-42. [Crossref] [PubMed]
- Park IK, Kim YT, Jeon JH, et al. Importance of lymph node dissection in thymic carcinoma. Ann Thorac Surg 2013;96:1025-32; discussion 1032. [Crossref] [PubMed]
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S65-72. [Crossref] [PubMed]
- Hsin MK, Keshavjee S. Should lymph nodes be routinely sampled at the time of thymoma resection? J Thorac Cardiovasc Surg 2015;149:743-4. [Crossref] [PubMed]
- Agasthian T. Can invasive thymomas be resected by video-assisted thoracoscopic surgery? Asian Cardiovasc Thorac Ann 2011;19:225-7. [Crossref] [PubMed]
- Sakamaki Y, Oda T, Kanazawa G, et al. Intermediate-term oncologic outcomes after video-assisted thoracoscopic thymectomy for early-stage thymoma. J Thorac Cardiovasc Surg 2014;148:1230-37.e1. [Crossref] [PubMed]
- Manoly I, Whistance RN, Sreekumar R, et al. Early and mid-term outcomes of trans-sternal and video-assisted thoracoscopic surgery for thymoma. Eur J Cardiothorac Surg 2014;45:e187-93. [Crossref] [PubMed]
- Marulli G, Rea F, Melfi F, et al. Robot-aided thoracoscopic thymectomy for early-stage thymoma: A multicenter European study. J Thorac Cardiovasc Surg 2012;144:1125-30. [Crossref] [PubMed]
- Ye B, Tantai JC, Li W, et al. Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery in the surgical treatment of Masaoka stage I thymoma. World J Surg Oncol 2013;11:157. [Crossref] [PubMed]
- Qian L, Chen X, Huang J, et al. A comparison of three approaches for the treatment of early-stage thymomas: robot-assisted thoracic surgery, video-assisted thoracic surgery, and median sternotomy. J Thorac Dis 2017;9:1997-2005. [Crossref] [PubMed]
- Wagner OJ, Louie BE, Vallières E, et al. Near-Infrared Fluorescence Imaging Can Help Identify the Contralateral Phrenic Nerve During Robotic Thymectomy. Ann Thorac Surg 2012;94:622-5. [Crossref] [PubMed]
- Toker A, Kaba E, Ayalp K, et al. Gentle traction on the superior poles and visualization of the thymic veins. Asvide 2018;5:402. Available online: http://www.asvide.com/article/view/24320
Cite this article as: Toker A, Kaba E, Ayalp K, Cosgun T, Alomari MR. Minimally invasive resection of thymoma. Mediastinum 2018;2:36.