
不可切除胸腺瘤诱导放化疗
大部分胸腺瘤在早期被发现,即 Masaoka-Koga I 或 II 期或 T1a-b2N0M0(建议的胸腺肿瘤第 8 版分期)[1],其中手术切除仍然是治愈性治疗的主要手段。大约1/3的胸腺瘤患者是局部晚期,临床分期为III期,综合治疗模式是其标准治疗。尽管缺乏随机临床研究,但诱导化疗在局部晚期疾病中已经广泛应用[2,3]。同步放化疗是一种有吸引力的治疗策略,因为其相互作用可以增加治疗疗效和提高手术完整切除率。对比术后瘤床区放疗,术前放疗需要更精准。有学者认为对于局部晚期胸腺瘤进行诱导放化疗会减少胸膜播散和局部复发率。
诱导放化疗在局部晚期或者不可切除胸腺的研究数据较少。最早的一项前瞻性多中心试验是某协作组在1977年发表的,该试验对局限不可切除胸腺瘤放化疗进行了评价[4]。这项研究分析了1983年-1995年积累的23名局部晚期胸腺瘤和胸腺癌患者[Masaoka-Koga III期(n=22),IV期(n=1)]肿瘤病灶和转移淋巴结情况。这些患者接受总剂量54Gy的调强放疗(IMRT)并序贯2-4周期的化疗(顺铂,多西他赛,环磷酰胺)。报道称单独化疗有效率为70%,放疗增加了部分患者(5/23)的完全或部分缓解率,未有患者进行手术治疗。在65%局部失败率病例中对比发现,四个周期化疗较两个周期化疗增加生存获益,作者报道此项研究的5年生存率为53%(表1)。
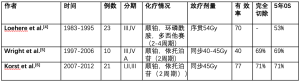
Full table
自从证实诱导化疗联合同步放疗提高了胸部肿瘤例如肺上沟瘤[7]或局部晚期远端食管癌[8]的手术完全切率,我们机构对初始治疗的胸腺瘤在手术前采用两周期化疗(顺铂,依托泊苷)同步总剂量40-45 Gy调强放疗。Wright和其他学者报道了从1997年到2006年积累的10例患者的回顾性研究数据[5]。此项研究中大部分患者(80%)肿瘤直径大于8公分,包含7名Masaoka-Koga III期患者,3名IVA期患者,仅1名患者为胸腺癌,放疗有效率为40%,这可能与化疗期间减少放疗剂量相关。所有患者在诱导治疗后4-8周都进行了手术,达到R0切除的患者为80%,没有患者获得完全病理缓解,然而,4个患者获得将近完全病理缓解。值得注意的是,PET扫描不能够反映出放疗有效率或是病理缓解率。此报道的中位随访时间为41个月,五年生存率为69%。
另外一项仍来自Wright等发表的回顾性、多中心、II期临床研究,研究采用2个周期诱导同步放化疗(顺铂+依托泊苷)联合45Gy放疗[6]。此项研究根据具体标准入组4个中心的21名患者,包含14名胸腺瘤和7名胸腺癌患者。放疗有效率为47%(在目前这些胸腺癌研究中最高)。完全切除率达77%,令人惊讶的是,这些人群未有完全病理缓解。相反,在一些报道中单独化疗完全缓解率为10%~15%[9-12]。较好的病理缓解率是否转化为生存获益仍有待证实。5名患者的肿瘤残留<10%(4名为胸腺癌)。9名患者(41%)出现3/4级毒性,2名患者出现术后死亡。此项研究5年生存率为71%。尽管诱导放化疗提高手术完整切除率,但是对比单独诱导化疗的优势却未明确。
除了肿瘤分期,在研究报道中R0手术切除仍然是最重要的预后因素。尽管联合放化疗提高胸腺瘤的手术完整切除率,但是却明显增加了治疗毒性,以及因为放疗的影响给手术区域的定位造成了困扰。对于局部晚期不可切除的胸腺瘤,应该通过诱导化疗以最大限度地提高完全切除的机会。如果外科医生已经在无法安全地实现完全切除的情况下进行手术,则有必要尽可能减少肿瘤体积,平衡相邻结构的风险和术后并发症,同时减少其他惰性肿瘤中的肿瘤体积。在一项荟萃分析中,将不完全切除术与观察到的“不可切除”胸腺瘤相比较,减瘤术与改善总生存率相关[13]。然后外科医生应该留下夹子以促进术后定向放射治疗。
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors Mirella Marino and Brett W. Carter for the series “Dedicated to the 8th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2017)” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2017.10.05). The series “Dedicated to the 8th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2017)” was commissioned by the editorial office without any funding or sponsorship. This study has been presented at 8th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2017). The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S65-72. [Crossref] [PubMed]
- Lemma GL, Lee JW, Aisner SC, et al. Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma. J Clin Oncol 2011;29:2060-5. [Crossref] [PubMed]
- Ahmad U, Huang J. Induction Therapy for Thymoma. Thorac Surg Clin 2016;26:325-32. [Crossref] [PubMed]
- Loehrer PJ Sr, Kim K, Aisner SC, et al. Cisplatin plus doxorubicin plus cyclophosphamide in metastatic or recurrent thymoma: final results of an intergroup trial. The Eastern Cooperative Oncology Group, Southwest Oncology Group, and Southeastern Cancer Study Group. J Clin Oncol 1994;12:1164-8. [Crossref] [PubMed]
- Wright CD, Choi NC, Wain JC, et al. Induction chemoradiotherapy followed by resection for locally advanced Masaoka stage III and IVA thymic tumors. Ann Thorac Surg 2008;85:385-9. [Crossref] [PubMed]
- Korst RJ, Bezjak A, Blackmon S, et al. Neoadjuvant chemoradiotherapy for locally advanced thymic tumors: a phase II, multi-institutional clinical trial. J Thorac Cardiovasc Surg 2014;147:36-44, 46.e1.
- Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Clin Oncol 2007;25:313-8. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Lucchi M, Melfi F, Dini P, et al. Neoadjuvant chemotherapy for stage III and IVA thymomas: a single-institution experience with a long follow-up. J Thorac Oncol 2006;1:308-13. [Crossref] [PubMed]
- Kunitoh H, Tamura T, Shibata T, et al. A phase II trial of dose-dense chemotherapy, followed by surgical resection and/or thoracic radiotherapy, in locally advanced thymoma: report of a Japan Clinical Oncology Group trial (JCOG 9606). Br J Cancer 2010;103:6-11. [Crossref] [PubMed]
- Kim ES, Putnam JB, Komaki R, et al. Phase II study of a multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation therapy, and consolidation chemotherapy for unresectable malignant thymomas: final report. Lung Cancer 2004;44:369-79. [Crossref] [PubMed]
- Hamaji M, Ali SO, Burt BM. A meta-analysis of induction therapy for advanced thymic epithelial tumors. Ann Thorac Surg 2015;99:1848-56. [Crossref] [PubMed]
- Hamaji M, Kojima F, Omasa M, et al. A meta-analysis of debulking surgery versus surgical biopsy for unresectable thymoma. Eur J Cardiothorac Surg 2015;47:602-7. [Crossref] [PubMed]

王璐
硕士研究生毕业南昌大学医学院,江西省整合医学会肿瘤精准诊疗分会委员会委员,江西省整合医学会肿瘤免疫治疗分会委员会委员2017年到上海复旦大学肿瘤医院肿瘤科。擅长肺部,乳腺癌及胸部肿瘤内科治疗,致力癌痛规范化及姑息病房建设。(更新时间:2021/9/23)
(本译文仅供学术交流,实际内容请以英文原文为准。)
Cite this article as: Lanuti M. Induction chemoradiotherapy for unresectable thymic tumors. Mediastinum 2017;1:13.



