The evolution of the histopathologic classification of thymic epithelial tumors
General considerations—embryologic uncertainty
Until the second half of the 20th century, the role of the thymus was largely unknown. Therefore, the microscopic observations on thymic tumors, with no awareness of structure and physiology of this organ, initially included several other tumors. These tumors included neoplasms with variable behaviour, some of them characterized by aggressive behaviour. This led to confusing ideas concerning the neoplastic growth of the thymus. As far as embryology is concerned, 2 different theories on the lymphocytic thymic content were discussed at the beginning of the 20th century. According to the immigration theory, sustained by Alexander A. Maximow (Figure 1) at about the second month of prenatal life, the thymus, which up to this time has been an endodermal organ, begins to being infiltrated by lymphoid cells of mesenchymal origin, which proliferate within the thymus and differentiate into lymphocytes. The lymphocytes separate the epithelial cells (EC) of the thymus (1,2). On the other hand, the “monistic” school, headed by James Ewing (Figure 2), believed that the endodermal cells themselves (endodermal thymic reticulum) differentiate into so-called thymic (lymphatic) cells (transformation theory) (3-5).


The first descriptions and the term “thymoma”
Among the first reports of morphologic descriptions of thymic tumors, J. Paviot and E. Gerest in 1896 (6) described a large mediastinal tumor which they named “epithelioma” of the thymus. This tumor presented at autopsy with a metastatic nodule outside the thorax (in the kidney capsule) in a patient who had died of asphyxia. In addition to cords of ECs, the tumor contained spherical bodies which appeared to have been derived from EC (7). Similar bodies had already been described by Arthur Hill Hassall in 1846 (8) and later named “Hassall’s bodies” or corpuscles (HC).
The term “thymoma” was first introduced by F. Grandhomme in 1900 (9). At that time, it was applied to all malignant tumors arising in the thymic gland. In 1906 E. T. Bell (10) first described tumors of the thymus that were associated with myasthenia gravis (MG) and used the term “thymoma” meaning non-malignant tumors. Later on, in 1939 A. Blalock et al. (11) summarized the confusing nomenclature of “thymoma”: “Decker (12) stated: by thymoma, Brown means carcinoma; Crotti all tumors; Bell non-malignant tumors; Margolis all tumors of parenchymal origin.”
Early classifications and the “granulomatous thymoma concept”
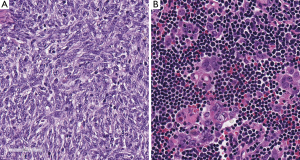
Among the first relevant classifications of thymic tumors, one has to mention the classifications by James Ewing [1928] (Figure 2) (4), Douglas Symmers [1932] (13), and Elizabeth Lowenhaupt [1948] (14) (Table 1). Some of these early classifications were based on morphological characteristics such as the predominance of lymphocytes or epithelial elements. Others were focused on the apparent origin of the tumors (parenchyma vs stroma of the thymic gland). Lowenhaupt’s classification, which tentatively correlated the morphology of thymic tumors with embryology, was of interest because it included the entity of “granulomatous thymoma” (15). However, the “granulomatous thymoma” was later considered “a misconception”, because it represented in fact a …. Hodgkin lymphoma (16,17) (Figure 3)! An extraordinary description of the History of Hodgkin’s disease in the thymus was reported by A.D. Thomson in 1955 (18) (Figure 4)

Full table

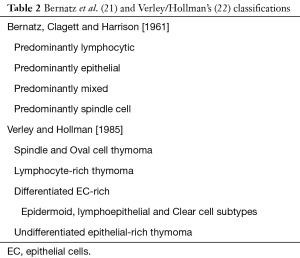
Development of the thymoma classification: the search for morphological categories and prognostic features
In the second part of the 20th century, several classification schema were proposed, along with the development of cellular immunology after the discoveries of RA Good (19) and JF Miller (20) on the fundamental role of Thymus and its cellular components. The collaboration between two thoracic surgeons (PE Bernatz and O. Clagett) and a pathologist (E. Harrison), at the Mayo Clinic in Rochester, Minnesota, produced a simple, and exclusively morphologic classification schema based on the predominant cellular type (epithelial or lymphocytic) and their relative numbers (Table 2) (21). The Verley and Hollman’s schema, later proposed in 1985 (22) was strictly related to the Bernatz proposal (it recognized four types: spindle cell, lymphocyte rich, and the epithelial-cell rich type was subdivided into differentiated and undifferentiated types) but these researchers added a prognostic value to their schema, classifying thymoma types from benign to potentially aggressive. Invasiveness and histologic typing appeared as two distinct parameters with separate prognostic significance, particularly in differentiated and undifferentiated epithelial tumors (Table 2).
Raffaele Lattes from Turin, Italy, immigrated to the United States and later established at Columbia University, in 1962 firmly established the epithelial nature of “reticulum cells” of the thymus. He proposed a morphological classification based on the EC type and lymphocytic content (Table 3) and together the reappraisal of the encapsulation or “non encapsulation” of the tumor. In addition, he noticed the association between MG with the Lymphocytic type, and of Aregenerative anemia with the Spindle cell type. Moreover, he discussed the problem of the malignancy of thymoma (15). Previously, Benjamin Castleman, who also contributed a relevant study on Tumors of the thymus gland in the First Series of the Armed Forces Institute of Pathology Atlas of tumor pathology (23), argued that, “since thymomas do not spread by embolic metastases, they should not be considered malignant even if they sometimes recur after surgery or seed themselves as multiple pleural implants or extend and penetrate into adjacent structures”. Later on, Juan Rosai and Gerald D. Levine investigated together the ultrastructural features of EC in thymoma. They firmly established the epithelial nature of the “thymoma”. Their study was reflected in the 1976 edition (second series) of the Fascicle of the Armed Forces Institute of Pathology, representing the first and best organized book on thymic tumors (24). The authors established that “once the term thymoma is restricted to the tumor of epithelial thymic cells, with or without a lymphocytic component, all further subdivisions are artificial”.
Subsequently [1978] Levine and Rosai, respectively from the Stanford University, Stanford and the University of Minnesota, Minneapolis, US, proposed a classification concerning the histologic aspect of the tumour and the biological behaviour as determined by the degree of invasion at surgery (Table 4).They classified thymic epithelial tumors (TET) into benign encapsulated thymoma, type I malignant thymoma (invasive thymoma), and type II malignant thymoma (thymic carcinoma). They did not attempt to subtype TET. A definite statement of the benign nature of lymphocytes in thymoma was made (25). Although the neoplastic nature of the EC and the benign properties of lymphocytes in TET have been now well accepted (26) it should be kept in mind that, in contrast to other tumors, most lymphocytes in thymoma are the expression of the thymopoietic capability of EC and not reactive lymphocytes.
During the same period [1981], professor Akira Masaoka from the Nagoya University, Japan, published his staging system for thymoma, based on a series of 81 cases (27). This world-wide widely used system considered the prognostic value of the tumor capsule. The Masaoka staging system, and the subsequent modification by Kenji Koga et al. (28) (Figure 5) required the key role of pathologists in the microscopic evaluation of the tumor.

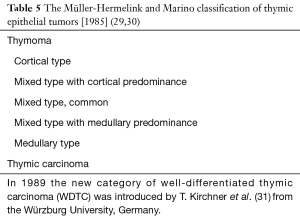
The so called “histogenetic” European classification (29,30) proposed by Prof. HK Müller-Hermelink and coworkers from the University of Würzburg, Germany, derived from the consideration of morphological similarities of EC in thymoma from the cortical and medullary compartments of the thymic gland (Table 5). Thymic carcinomas were defined by the presence of invasive growth and almost pure composition of EC with cytologic criteria of malignancy. However, whereas the resemblance of the large dendritic-like cells of the thymoma to the cortical EC was more obvious, the concept of medullary EC was more criticized.
Full table
A separate category of well-differentiated thymic carcinoma (WDTC) was later recognized by HK Müller-Hermelink and T. Kirchner (31) and was added to the original “histogenetic” classification as a low-grade malignancy. In WDTC, EC with mild to moderate atypia predominated and showed an epidermoid or squamoid growth pattern with or without keratinization. An organotypic thymic feature was recognizable by its lobular growth pattern and the presence of perivascular spaces (PVS). The prognostic value of this “histogenetic” classification has been confirmed in several studies (32,33), but questioned in others (34). Moreover, several papers focused on pros- (35) and cons- (36) interobserver reproducibility of this histological classification.
The 1999 WHO classification—the value of cytoarchitectural features of TET
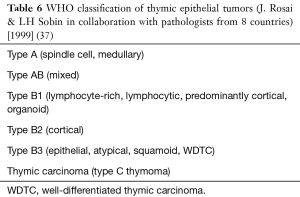
The WHO classification of 1999 was the result of several years of work by a panel of 8 pathologists, coordinated by Prof. Rosai (37) (Table 6). It represented a compromise among different views on thymoma classification. In our opinion, however, it was basically derived from the concept of the “histogenetic” classification (29). The first edition of the WHO classification emphasized that the classification of cytoarchitectural features of thymoma should occur independently of staging. TETs were classified according to the number and shape of EC and the number of lymphocytes in the tumor. The use of two alphabetic letters (A and B) made it possible to identify as “A”, tumors with a component of spindle-oval EC without lymphocytes, and as “B”, tumors with a component of large EC with dendritic (epithelioid) morphology, forming a lymphocyte attracting network. Tumours combining these two morphologies were designated as type AB. “Type B” thymomas were further subdivided into three subtypes on the basis of the proportional increase (in relation to the lymphocytes) and emergence of atypia of neoplastic EC, respectively designated as B1, B2 and B3. According to Prof. Rosai, “A” stands for atrophic (i.e., the effete thymic EC of adult life), “B” for bioactive (i.e., the biologically active organ of the fetus and infant) (Figure 6). The validity of the 1999 WHO classification has been confirmed in several studies) (39). Particularly, a large-scale report of TET stated that overall survival (OS) rates for patients with type A, AB, or B1 tumors were higher than those for patients with type B2 or B3 tumors, therefore pointing to the definition of “prognostic” groups (38).
Full table
The Suster & Moran proposal—the value of cellular atypia
Saul Suster and Cesar A. Moran, respectively at the Ohio State University, Columbus and the University of Alabama at Birmingham, US, were opponents of Müller-Hermelink’s histogenetic classification and of the subsequent WHO classification editions of TET (40). In 1999, the same year in which the first WHO classification of TET was published, they proposed to classify TET as thymoma (well differentiated thymic epithelial neoplasm), atypical thymoma (moderately differentiated thymic epithelial neoplasm), and thymic carcinoma (poorly differentiated thymic epithelial neoplasm) (41) (Table 7). The “atypical thymoma” actually corresponded to the “WDTC” of the Müller-Hermelink classification. The cellular atypia was the basis of their proposal, and they lumped together types A-AB, B1 and B2 of the WHO classification in the “thymoma” group, leaving only the B3 type in the “atypical thymoma” group. Organotypical features of the “thymoma” group included lobulation, an admixture of immature T cells and EC, foci of medullary differentiation and PVS. Clinicopathological data support the interest and value of this classification (42).

Full table
Progressing strategies—the 2004 and 2015 WHO classification
The WHO edition of TET classification published in 2004 (43) reflected a renewed view of the pathological diagnosis, considering the clinical symptoms of patients, the macroscopic findings of the tumor, immunohistochemical and genetic features and prognostic data. Type C thymoma of the 1999 WHO classification was termed as thymic carcinoma, while the morphologic subtypes of thymomas remained unchanged. In the following years, the WHO-based histological thymoma subtyping proved to be significant for the OS of thymoma patients (44,45). Moreover, the WHO classification proved to be an independent prognostic factor, with 10-year disease-free survival (DFS) rates decreasing from A, AB, B1, B2 to B3, respectively. An early-stage, low-risk group of types A, AB and B1 thymoma was recognized (46,47). Genetic support by comparative genomic hybridization and microsatellite analysis to the WHO subtyping was provided by a series of studies—most of them from the Würzburg group—across the full spectrum of WHO types (48-50). These studies provided evidence that B3 thymoma and thymic carcinoma are strongly related through their chromosomal imbalances and that, with few exceptions, type A tumours lack chromosomal gains or losses.
In the period between the two WHO classification revisions, from the 2004 to 2015, the International Thymic Malignancy Interest Group (ITMIG) (www.itmig.org) was established in 2010 and was able to engage a worldwide community, which supported the development of a centralized database (51,52). Therefore, demographic, epidemiologic, pathological and prognostic data for the 2015 WHO classification (53) were provided. Moreover, the new 2015 edition of the WHO classification established an interdisciplinary approach to the diagnosis of TET, by sharing the study with radiologists, thoracic surgeons and oncologists (Table 8, Figures 7 and 8). Some histomorphologic features and immunohistochemical criteria were refined during two pathology workshops held in New York, US and in Mannheim, Germany in order to enhance the reproducibility of thymoma subtyping and to facilitate the distinction between thymomas and thymic carcinomas (54). A short summary of refined diagnostic criteria in TET subtyping is provided:
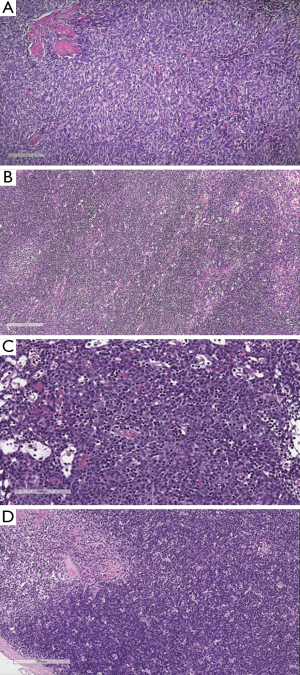
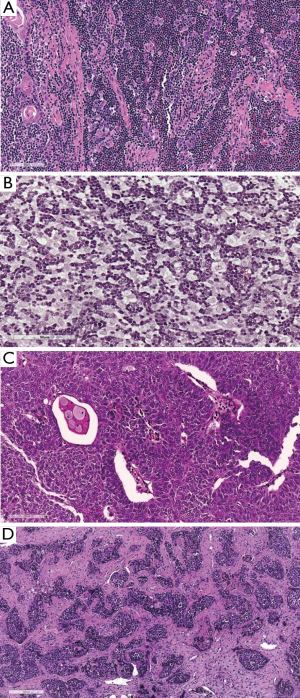
- Type A was distinguished from AB by a “low throughout” or “focally moderate” content of immature, TDT+ T cells (Figure 7A vs. Figure 7B and Figure 7C);
- Type B1 and B2 were redefined on H & E by either absence (B1) or presence (B2) of EC clustering (Figure 7D vs. Figure 8A and Figure 8B);
- The B2 versus B3 distinction remained an H & E diagnosis (blue vs. pink) (Figure 8A and 8B vs. Figure 8C).
The never ending history of TET—recent developments
Although, according to Lalla Iverson (55) “neoplasia of the thymus gland displays such freedom of expression that it conforms to none but the broadest of definitions”, considerable progress in TET classification has been made during the last century. Further developments are now ongoing in order to provide patients with meaningful pathological data. The refinement of morphological approaches is expected to progress in conjunction with detailed clinical data and new relevant genetic and epigenetic findings.
Acknowledgments
The authors thank Professor HK Müller-Hermelink, Würzburg, Germany, for reading the manuscript; M. Marino would like to thank Dr. Michael Kenyon for his review of the English language, and Dr. Enzo Gallo for photographical assistance.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Mediastinum for the series “Diagnostic Problems in Anterior Mediastinum Lesions” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2018.01.04). The series “Diagnostic Problems in Anterior Mediastinum Lesions” was commissioned by the editorial office without any funding or sponsorship. MM serves as an unpaid editorial board member of Mediastinum from May 2017 to Apr 2019. ACR serves as an unpaid Associate Editor of Mediastinum from May 2017 to Apr 2019. Part of the data had been presented at the 8th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2017), September 21–23, 2017, Torino, Italy. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Maximow AA. Untersuchungen über Blut und Bindegewebe. II Über die Histogenese der Thymus bei Säugetieren. Arch Mikr Anat (Bonn) 1909;74:525-621. [Crossref]
- Hammar JA. Fünfzig Jahre Thymusforschung: Kritische Übersicht der normalen Morphologie. Ergebn Anat Entwickl Gesch (Wiesbaden) 1910;19:1-274.
- Ewing J. Thymus and its tumors. Report of 3 cases of thymoma. Surg Gynec & Obst 1916;22:461-72.
- Ewing J. Neoplastic diseases: a treatise on tumors. 3rd edition. Philadelphia: Saunders, WB; 1928.
- Crosby EH. Malignant tumors of the thymus gland. Am J Cancer 1932;16:461-86.
- Paviot J, Gerest E. Un cas J'epithclioma primitif du thymus. Arch de med exper et J'anat path 1896;8:606.
- Margolis H. Tumors of the Thymus: Pathology, Classification and Report of cases. Cancer Res 1931;15:2106-42.
- Hassall AH. The microscopic anatomy of the human body, in health and disease. Illustrated with numerous drawings in colour. London: Samuel Highley, 1849.
- Grandhomme F. Über Tumoren des vorderen Mediastinums und ihre Beziehungen zu der Thymusdrüse. Heidelberg: Inaug Diss, 1900.
- Bell ET. Tumors of the thymus in myasthenia gravis. J Nerv Ment Dis 1917;42:130. [Crossref]
- Blalock A, Mason M, Morgan H, et al. Myasthenia gravis and tumors of the thymic region: Report of a case in which the tumor was removed. Ann Surg 1939;110:544-61. [Crossref] [PubMed]
- Decker H. Primary malignant tumors of the thymus gland. Jour Thoracic Surg 1935;4:445.
- Symmers D. Malignant Tumors and Tumor-like Growths of the Thymic Region. Ann Surg 1932;95:544-72. [Crossref] [PubMed]
- Lowenhaupt E. Tumors of the thymus in relation to the thymic epithelial anlage. Cancer 1948;1:547-63. [Crossref]
- Lattes R. Thymoma and other tumors of the thymus: an analysis of 107 cases. Cancer 1962;15:1224-60. [Crossref]
- Katz A, Lattes R. Granulomatous thymoma or Hodgkin's disease of thymus? A clinical and histologic study and a re-evaluation. Cancer 1969;23:1-15. [Crossref] [PubMed]
- Rosai J. Lowenhaupt’s embryology-based classification of thymic tumors and the concept of granulomatous thymoma. Cancer 1998;82:1209-16. [Crossref] [PubMed]
- Thomson AD. The thymic origin of Hodgkin’s disease. Br J Cancer 1955;9:37-50. [Crossref] [PubMed]
- Good RA, Maclean L, Varco R, et al. Thymic tumor and acquired agammaglobulinemia: a clinical and experimental study of the immune response. Surgery 1956;40:1010-7. [PubMed]
- Miller JF. Immunological function of the thymus. Lancet 1961;2:748-9. [Crossref] [PubMed]
- Bernatz PE, Harrison EG, Clagett OT. Thymoma: a clinicopathologic study. J Thorac Cardiovasc Surg 1961;42:424-44. [PubMed]
- Verley JM, Hollmann KH. Thymoma. A comparative study of clinical stages, histologic features, and survival in 200 cases. Cancer 1985;55:1074-86. [Crossref] [PubMed]
- Castleman B. Tumors of the thymus gland First series edition. Atlas of Tumor pathology, vol 19. Washington, DC: Armed Forces Institute of Pathology, 1955.
- Rosai J, Levine GD. Tumors of the Thymus. Second series edition. Atlas of tumor Pathology, vol 13. Washington, DC: Armed Forces Institute of Pathology, 1976.
- Levine GD, Rosai J. Thymic hyperplasia and neoplasia: a review of current concepts. Hum Pathol 1978;9:495-515. [Crossref] [PubMed]
- Roden AC. Evolution of Classification of Thymic Epithelial Tumors in the Era of Dr Thomas V. Colby. Arch Pathol Lab Med 2017;141:232-46. [Crossref] [PubMed]
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [Crossref] [PubMed]
- Koga K, Matsuno Y, Noguchi M, et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int 1994;44:359-67. [Crossref] [PubMed]
- Marino M, Müller-Hermelink HK. Thymoma and thymic carcinoma. Relation of thymoma epithelial cells to the cortical and medullary differentiation of thymus. Virchows Arch A Pathol Anat Histopathol 1985;407:119-49. [Crossref] [PubMed]
- Müller-Hermelink HK, Marino M, Palestro G, et al. Immunohistological evidences of cortical and medullary differentiation in thymoma. Virchows Arch A Pathol Anat Histopathol 1985;408:143-61. [Crossref] [PubMed]
- Kirchner T, Schalke B, Buchwald J, et al. Well-differentiated thymic carcinoma. An organotypical low-grade carcinoma with relationship to cortical thymoma. Am J Surg Pathol 1992;16:1153-69. [Crossref] [PubMed]
- Pescarmona E, Rendina EA, Venuta F, et al. The prognostic implication of thymoma histologic subtyping. A study of 80 consecutive cases. Am J Clin Pathol 1990;93:190-5. [Crossref] [PubMed]
- Ho FC, Fu KH, Lam SY, et al. Evaluation of a histogenetic classification for thymic epithelial tumours. Histopathology 1994;25:21-9. [Crossref] [PubMed]
- Kornstein MJ, Curran WJ, Turrisi AT, et al. Cortical versus medullary thymomas: a useful morphologic distinction? Hum Pathol 1988;19:1335-9. [Crossref] [PubMed]
- Close PM, Kirchner T, Uys CJ, et al. Reproducibility of a histogenetic classification of thymic epithelial tumours. Histopathology 1995;26:339-43. [Crossref] [PubMed]
- Shimosato Y, Mukai K. Tumors of the Mediastinum, Atlas of tumor pathology Atlas of Tumor pathology, vol Third series. Washington D.C.: Armed Forces Institute of Pathology, 1997.
- Rosai J, Sobin LH. Histological typing of tumours of the thymus. World Health Organization. International histological classification of tumours. 1999.
- Chen G, Marx A, Chen WH, et al. New WHO histologic classification predicts prognosis of thymic epithelial tumors: a clinicopathologic study of 200 thymoma cases from China. Cancer 2002;95:420-9. [Crossref] [PubMed]
- Okumura M, Ohta M, Tateyama H, et al. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: a clinical study of 273 patients. Cancer 2002;94:624-32. [Crossref] [PubMed]
- Moran CA, Suster S. The World Health Organization (WHO) histologic classification of thymomas: a reanalysis. Curr Treat Options Oncol 2008;9:288-99. [Crossref] [PubMed]
- Suster S, Moran CA. Thymoma, atypical thymoma, and thymic carcinoma. A novel conceptual approach to the classification of thymic epithelial neoplasms. Am J Clin Pathol 1999;111:826-33. [Crossref] [PubMed]
- Weissferdt A, Kalhor N, Bishop JA, et al. THYMOMA: A clinicopathological correlation of 1470 cases. Hum Pathol 2017; [Epub ahead of print]. [PubMed]
- Travis WD, Brambilla E, Müller-Hermelink HK, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARCPress, 2004.
- Ströbel P, Bauer A, Puppe B, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol 2004;22:1501-9. [Crossref] [PubMed]
- Roden AC, Yi ES, Jenkins SM, et al. Diagnostic significance of cell kinetic parameters in World Health Organization type A and B3 thymomas and thymic carcinomas. Hum Pathol 2015;46:17-25. [Crossref] [PubMed]
- Park MS, Chung KY, Kim KD, et al. Prognosis of thymic epithelial tumors according to the new World Health Organization histologic classification. Ann Thorac Surg 2004;78:992-7; discussion 7-8. [Crossref] [PubMed]
- Rena O, Papalia E, Maggi G, et al. World Health Organization histologic classification: an independent prognostic factor in resected thymomas. Lung Cancer 2005;50:59-66. [Crossref] [PubMed]
- Inoue M, Starostik P, Zettl A, et al. Correlating genetic aberrations with World Health Organization-defined histology and stage across the spectrum of thymomas. Cancer Res 2003;63:3708-15. [PubMed]
- Penzel R, Hoegel J, Schmitz W, et al. Clusters of chromosomal imbalances in thymic epithelial tumours are associated with the WHO classification and the staging system according to Masaoka. Int J Cancer 2003;105:494-8. [Crossref] [PubMed]
- Zettl A, Ströbel P, Wagner K, et al. Recurrent genetic aberrations in thymoma and thymic carcinoma. Am J Pathol 2000;157:257-66. [Crossref] [PubMed]
- Huang J, Ahmad U, Antonicelli A, et al. Development of the international thymic malignancy interest group international database: an unprecedented resource for the study of a rare group of tumors. J Thorac Oncol 2014;9:1573-8. [Crossref] [PubMed]
- Detterbeck FC, Asamura H, Crowley J, et al. The IASLC/ITMIG thymic malignancies staging project: development of a stage classification for thymic malignancies. J Thorac Oncol 2013;8:1467-73. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th edition ed. Lyon: International Agency for Research on Cancer-IARC- press, 2015.
- Marx A, Ströbel P, Badve SS, et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Oncol 2014;9:596-611. [Crossref] [PubMed]
- Iverson L. Thymoma; a review and reclassification. Am J Pathol 1956;32:695-719. [PubMed]
Cite this article as: Marino M, Roden AC. The evolution of the histopathologic classification of thymic epithelial tumors. Mediastinum 2018;2:9.