
Extended surgical resection for stage III thymic tumors
Introduction
Thymic tumors (TTs) are rare malignancies accounting for 0.2–1.5% of all tumors (1) and thymoma represents the most common histology (~90% of all resected neoplasms) (2). Masoka-Koga stage III TTs occurs in 20–29% of all surgically treated thymomas (2,3). It is a heterogeneous entity characterized by a variety of locally-advanced neoplasms with macroscopic invasion of different intrathoracic structures (pericardium, lung, great vessels, phrenic nerve, diaphragm, chest wall) (4) as well as a different prognosis according to specific WHO histologic subtypes (5). This high variability in clinicopathologic behavior has long represented a determent for most clinicians in evaluating the optimal therapeutic strategy in such tumors. In order to overcome this heterogeneity, the new TNM staging system has divided the subset of locally-advanced TTs in three different stages according to the invasion of pericardium (stage II), neighbor organs (stage IIIa) or “unresectable” ones (stage IIIb) (6). Nevertheless, treatment of locally-advanced disease is still challenging in terms of surgical resectability as well as multimodality therapies adopted to improve survival and to control recurrences (7).
Outcome
From the surgical point of view, stage III TTs are subjected to a different range of radical resections (2,3,8). These data are of particular interest if considering that overall survival decreases with higher stages (III–IV) as well as incomplete resection, and recurrence rate increases in case of greater tumor size, stages III–IV, and more aggressive histologic features (2,9).
In the literature, few studies only have analyzed the subset of stage III TTs. Specifically, the 5-year overall survival still remains acceptable after surgery, ranging from 82% in Italy (10), 82.8% in Europe (7), ~72% in USA (11) and ~86% in Japan (12) (78% in our Institution). In major surgical series, different prognostic factors have been analyzed in outcome analysis, being completeness of resection (7,10), age (7,11,12) and administration of adjuvant therapy (AT) (7,11) the most significant ones. Similarly to other surgical series analyzing all stages, completeness of resection has been reported as the best prognosticator (9,13), confirming the role of surgery as the mainstay of treatment in such disease. On the other hand, given the technical difficulties encountered by surgeons in locally-advanced TTs (for large tumor dimension or involvement of vital mediastinal organs), the reported rate of radical resection is still highly variable among different studies (from 50% to 81.6% of all resected tumors) (2,3,7,8,10). This issue is of particular interest if considering that incomplete resections or debulking procedures may lead to increased recurrence rates and poorer prognosis and could not improve survival compared to biopsy alone (14).
Pattern of recurrence
Although prognosis seems acceptable, the recurrence rate is not negligible in surgically-treated stage III TTs. Specifically, even after R0 resection, relapse occurs in 17.5% to 30% of locally-advanced tumors (7,15,16) being the pleura and the tumor bed the most common sites (3,7). The European Society of Thoracic Surgeons (ESTS) evidenced that larger tumors (greater than 5 cm) are more likely to be associated with local recurrence than with distant recurrence (7). Furthermore, a recurrence analysis performed on the Japanese Nationwide Database documented that patients with specific pattern of invasion (pericardium, phrenic nerve, and pleura) were more likely to have relapse in the pleural or pericardial cavity (12,15).
Role of extended resections
In the setting of multidisciplinary treatment of TTs, the decision on whether performing extended resection to obtain R0 resection is widely debated, particularly in the setting of stage III TTs with invasion of “vital” organs. In the literature, the surgical series reporting “very extended” procedures (i.e., superior vena cava or diaphragmatic resections) are limited (Table 1). With exception of pericardium, the main resected organs in extended thymectomy are the great vessels, ranging from 8.9% to 24.1% (24.8% in our experience) of all procedures (7,10), and specifically the brachiocephalic veins, the superior vena cava (17-22) and, even rarely, the aorta and epiaortic vessels (23). As reported in Table 1, the invasion of great vessels is associated with worse overall survival (ranging from 45% to 56% at 5 years) compared to those patients with invasion of lung, pericardium or phrenic nerve, even in case of R0 resection (10). The reason for poor outcome in this subset of patients is probably related to their higher risk of systemic recurrences, which are generally not suitable for a redo-resection (10,24). However, although the prognosis in stage III TTs with invasion of mediastinal organs seems unexceptional, the surgeon should avoid any debulking procedures and be able to perform extensive resections (and reconstruction as well) even in case of challenging cases with invasion of “vital” structures (i.e., vena cava, phrenic nerve or lung). Thus, it is highly recommended to manage TTs in high-volume centers and to resect all the organs close to the tumor (i.e., pericardium), even when the clinical staging is lower than stage III.
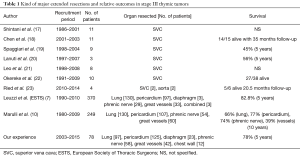
Full table
Induction therapy (IT)
In the setting of challenging cases, given the well documented chemo and radio-responsiveness of TTs, a variety of multimodality therapies has been employed through the years in order to improve prognosis and control relapse. Concerning administration of IT, few studies or clinical trials have been reported in the literature up to now (25-27), and even less in the setting of stage III alone (7,28). Reportedly, complete resection rate after IT varies between 22% and 92% (29), while 5-year OS approaches 80% (7,28). Although these data may suggest that resectability and survival is acceptable after IT in patients with locally-advanced disease (29), deeper analyses performed by the ESTS and the Japanese Nationwide Database evidenced that IT did not seem to affect survival (7) or may be an adverse prognostic factor as well (12), respectively. This is because IT is administered mainly to patients with more advanced disease [worse WHO histologic type, higher rate of incomplete resection (7), larger radiologic tumor size, higher number of involved sites, and invasion of the phrenic nerve (12)]. Furthermore, randomized trials on IT are hard to perform because of the following reasons: (I) TTs are extremely rare; (II) there is no appropriate comparison group (i.e., patients with potentially unresectable thymoma undergoing upfront surgery) or way to perform a propensity-score match for patients undergoing IT (7). Thus, the prognostic impact of IT is still controversial, and probably underpowered. Its real estimation would need further efforts in the setting of large prospective database.
AT
Contrarily to IT, the administration of AT is less debated in common practice. In the literature, some authors advocated no survival advantage in those patients receiving postoperative therapy (13,30). On the other hand, further analyses on larger databases have documented more positive results, especially in the setting of locally-advanced tumors. While a study on the SEER database evidenced improved disease-free survival only (11), a propensity-score match analysis performed on the ESTS database revealed that administration of AT was beneficial in terms of overall and cancer-specific survival either in stage III thymoma either in patients with specific pathologic features (7). These data have been also confirmed in a recent meta-analysis (31) as well as an investigation on 4,056 resected TTs enrolled in the National Cancer Data Base (32). Further studies are needed to define the optimal postoperative therapeutic strategy in the setting of locally-advanced TTs.
Conclusions
The management of stage III TTs is still challenging and constantly evolving. In order to provide the best chance to survival, all cases should be managed by a multidisciplinary team in high volume centers. IT may help the surgeon to obtain radicality in difficult cases with invasion of “unresectable” structures. On the other hand, the surgeon should avoid any debulking procedures and resect all the organs close to the tumor (even if the clinical staging is lower than stage III) or be able to perform extensive combined resections and reconstruction in more advanced cases. Further efforts should be made to evaluate the optimal combination of multimodality therapies in locally-advanced TTs.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors Mirella Marino and Brett W. Carter for the series “Dedicated to the 8th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2017)” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2018.04.04). The series “Dedicated to the 8th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2017)” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Travis WD, Brambilla E, Muller-Hermelink HK, et al. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart (World Health Organization Classification of Tumours). Lyon: IARC Press, 2004.
- Ruffini E, Detterbeck F, Van Raemdonck D, et al. Tumours of the thymus: a cohort study of prognostic factors from the European Society of Thoracic Surgeons database. Eur J Cardiothorac Surg 2014;46:361-8. [Crossref] [PubMed]
- Myojin M, Choi NC, Wright CD, et al. Stage III thymoma: pattern of failure after surgery and postoperative radiotherapy and its implication for future study. Int J Radiat Oncol Biol Phys 2000;46:927-33. [Crossref] [PubMed]
- Detterbeck FC, Nicholson AG, Kondo K, et al. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. J Thorac Oncol 2011;6:S1710-6. [Crossref] [PubMed]
- Marchevsky AM, Gupta R, Casadio C, et al. World Health Organization classification of thymomas provides significant prognostic information for selected stage III patients: evidence from an international thymoma study group. Hum Pathol 2010;41:1413-21. [Crossref] [PubMed]
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S65-72. [Crossref] [PubMed]
- Leuzzi G, Rocco G, Ruffini E, et al. Multimodality therapy for locally advanced thymomas: A propensity score-matched cohort study from the European Society of Thoracic Surgeons Database. J Thorac Cardiovasc Surg 2016;151:47-57.e1. [Crossref] [PubMed]
- Blumberg D, Port JL, Weksler B, et al. Thymoma: a multivariate analysis of factors predicting survival. Ann Thorac Surg 1995;60:908-13; discussion 914. [Crossref] [PubMed]
- Detterbeck FC, Parsons AM. Thymic tumors. Ann Thorac Surg. 2004;77:1860-9. [Crossref] [PubMed]
- Marulli G, Lucchi M, Margaritora S, et al. Surgical treatment of stage III thymic tumors: a multi-institutional review from four Italian centers. Eur J Cardiothorac Surg 2011;39:e1-7. [Crossref] [PubMed]
- Weksler B, Shende M, Nason KS, et al. The role of adjuvant radiation therapy for resected stage III thymoma: a population-based study. Ann Thorac Surg 2012;93:1822-8. [Crossref] [PubMed]
- Yamada Y, Yoshino I, Nakajima J, et al. Surgical Outcomes of Patients With Stage III Thymoma in the Japanese Nationwide Database. Ann Thorac Surg 2015;100:961-7. [Crossref] [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1320 patients from Japan. Ann Thorac Surg. 2003;76:878-84; discussion 884-5. [Crossref] [PubMed]
- Attaran S, Acharya M, Anderson JR, et al. Does surgical debulking for advanced stages of thymoma improve survival? Interact Cardiovasc Thorac Surg 2012;15:494-7. [Crossref] [PubMed]
- Haniuda M, Kondo R, Numanami H, et al. Recurrence of thymoma: clinicopathological features, re-operation, and outcome. J Surg Oncol 2001;78:183-8. [Crossref] [PubMed]
- Ruffini E, Mancuso M, Oliaro A, et al. Recurrence of thymoma: analysis of clinicopathologic features, treatment, and outcome. J Thorac Cardiovasc Surg 1997;113:55-63. [Crossref] [PubMed]
- Shintani Y, Ohta M, Minami M, et al. Long-term graft patency after replacement of the brachiocephalic veins combined with resection of mediastinal tumors. J Thorac Cardiovasc Surg 2005;129:809-12. [Crossref] [PubMed]
- Chen KN, Xu SF, Gu ZD, et al. Surgical treatment of complex malignant anterior mediastinal tumors invading the superior vena cava. World J Surg 2006;30:162-70. [Crossref] [PubMed]
- Spaggiari L, Leo F, Veronesi G, et al. Superior vena cava resection for lung and mediastinal malignancies: a single-center experience with 70 cases. Ann Thorac Surg 2007;83:223-9. [Crossref] [PubMed]
- Lanuti M, De Delva PE, Gaissert HA, et al. Review of superior vena cava resection in the management of benign disease and pulmonary or mediastinal malignancies. Ann Thorac Surg 2009;88:392-7. [Crossref] [PubMed]
- Leo F, Bellini R, Conti B, et al. Superior vena cava resection in thoracic malignancies: does prosthetic replacement pose a higher risk? Eur J Cardiothorac Surg. 2010;37:764-9. [Crossref] [PubMed]
- Okereke IC, Kesler KA, Rieger KM, et al. Results of superior vena cava reconstruction with externally stented-polytetrafluoroethylene vascular prostheses. Ann Thorac Surg 2010;90:383-7. [Crossref] [PubMed]
- Ried M, Neu R, Schalke B, et al. Radical surgical resection of advanced thymoma and thymic carcinoma infiltrating the heart or great vessels with cardiopulmonary bypass support. J Cardiothorac Surg 2015;10:137. [Crossref] [PubMed]
- Utsumi T, Shiono H, Matsumura A, et al. Stage III thymoma: relationship of local invasion to recurrence. J Thorac Cardiovasc Surg 2008;136:1481-5. [Crossref] [PubMed]
- Rea F, Marulli G, Di Chiara F, et al. Multidisciplinary approach for advanced stage thymic tumors: long-term outcome. Lung Cancer 2011;72:68-72. [Crossref] [PubMed]
- Korst RJ, Bezjak A, Blackmon S, et al. Neoadjuvant chemoradiotherapy for locally advanced thymic tumors: a phase II, multi-institutional clinical trial. J Thorac Cardiovasc Surg 2014;147:36-44, 46.e1.
- Park S, Ahn MJ, Ahn JS, et al. A prospective phase II trial of induction chemotherapy with docetaxel/cisplatin for Masaoka stage III/IV thymic epithelial tumors. J Thorac Oncol. 2013;8:959-66. [Crossref] [PubMed]
- Cardillo G, Lucchi M, Marulli G, et al. Induction therapy followed by surgical resection in Stage-III thimic epithelial tumors: Long-term results from a multicentre analysis of 108 cases. Lung Cancer 2016;93:88-94. [Crossref] [PubMed]
- Kondo K. Therapy for thymic epithelial tumors. Gen Thorac Cardiovasc Surg 2014;62:468-74. [Crossref] [PubMed]
- Korst RJ, Kansler AL, Christos PJ, et al. Adjuvant radiotherapy for thymic epithelial tumors: a systematic review and meta-analysis. Ann Thorac Surg 2009;87:1641-7. [Crossref] [PubMed]
- Lim YJ, Kim E, Kim HJ, et al. Survival Impact of Adjuvant Radiation Therapy in Masaoka Stage II to IV Thymomas: A Systematic Review and Meta-analysis. Int J Radiat Oncol Biol Phys 2016;94:1129-36. [Crossref] [PubMed]
- Jackson MW, Palma DA, Camidge DR, et al. The Impact of Postoperative Radiotherapy for Thymoma and Thymic Carcinoma. J Thorac Oncol 2017;12:734-44. [Crossref] [PubMed]
Cite this article as: Leuzzi G, Pastorino U. Extended surgical resection for stage III thymic tumors. Mediastinum 2018;2:40.


