
PTEN tumor suppressor expression in thymic epithelial tumors
Phosphatase and tensin homolog (PTEN) regulates cell proliferation and survival, cell metabolism and genomic stability (1). It constitutes a potent tumor suppressor (2). A loss of function is frequent in neoplastic diseases. Germline mutations of PTEN cause cancer susceptibility syndromes, such as Cowden’s disease (3). PTEN functions as a phosphatase for lipids, mainly the phosphatidylinositol (PtdIns)-3,4,5-triphosphate (PIP3). It thereby antagonizes the phosphatidylinositol-3-kinase (PI3K)/AKT/mTOR signaling pathway (Figure 1). PTEN, however, displays also non-enzymatic functions such as regulation of transcription and maintenance of genomic stability (1). The protein localizes to the cytoplasm and the nucleus (1,4).
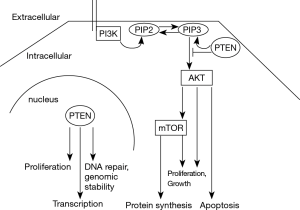
PTEN is frequently downregulated in malignant tumors. The mechanisms are inactivation by gene mutation or deletion, and transcriptional or translational silencing by promoter methylation and miRNA interference (1). A PTEN pseudogene (PTENP1) can also influence PTEN expression. The homology of its transcripts 3‘ untranslated region to PTEN allows it to quench PTEN-targeting miRNAs (5).
Masunaga et al. (6) analyzed the expression of PTEN and PTENP1 in thymomas and thymic carcinomas. They studied by immunohistochemistry the presence of PTEN protein with two different antibodies (clone 28H6 and polyclonal antiserum ab31392) in non-neoplastic thymus (n=2), type A (n=3), AB (n=8), B1 (n=11), B2 (n=6) and B3 thymomas (n=5) and thymic carcinomas (n=4). In sixteen cases (two non-neoplastic thymi, three A, five B1, two B2 and one B3 thymomas and three thymic carcinomas) additional PTEN exon sequencing, promoter methylation analysis and comparison of PTEN and PTENP1 transcript levels were performed with epithelial cells isolated by laser capture microdissection.
The authors hypothesized that epithelial cells of non-neoplastic thymus expressed PTEN protein and, inferring from the function of PTEN as a tumor suppressor, assumed that thymomas and thymic carcinomas might exhibit a loss of PTEN protein. In contrast to this expectation, PTEN protein was not detected in non-neoplastic thymic epithelial cells, except for a few cell nuclei of Hassall’s corpuscles. Furthermore, B1 and B2 thymoma cells did not express PTEN, and nuclear PTEN positivity was observed in only one of five B3 thymomas. By contrast, type A thymomas and thymic carcinoma cells did express PTEN protein. In type AB thymomas PTEN protein was present only in the type A component. Neither PTEN mutations nor promoter methylation were detected in any of the samples. PTEN mRNA expression was highest in non-neoplastic thymi and lowest in type A thymomas. PTENP1 transcript expression did not differ significantly among non-neoplastic thymus, thymoma and thymic carcinoma samples and is therefore an unlikely regulatory cause of the differences in PTEN expression. The authors speculated that non-neoplastic thymic epithelium and type B1/B2 thymoma cells possessed a mechanism of PTEN translational repression and/or acceleration of protein degradation, whereas type A thymoma cells exhibited transcriptional repression of PTEN and accelerated translation and/or protein accumulation (6).
A caveat of the study by Masunaga et al. (6) is the small number of analyzed samples. In particular, only two non-neoplastic thymi were studied. This restricts the meaningfulness of some of the results and conclusions offered. Furthermore, the authors describe the isolation of epithelial cells for RNA analysis by laser capture microdissection. However, the procedure is not described in detail and it is therefore unclear whether cell enrichment or single cell isolation was performed and how the purity of the samples was verified. This is a crucial issue as non-neoplastic thymi and B1/B2 thymomas in particular are rich in non-neoplastic lymphocytes. These lymphocytes, which strongly express PTEN protein, might have been a source of the relatively high PTEN mRNA content in the non-neoplastic thymi and B1/B2 thymomas. Conversely, type A thymomas contained few lymphocytes and exhibited low PTEN mRNA levels, but were immunohistochemically PTEN positive. Interestingly, thymic carcinomas contained also only few lymphocytes, but their PTEN mRNA levels were not significantly different from B1/B2 thymomas. The PTEN promoter methylation analysis was carried out with PCR primers previously described by Wiencke et al. (7). The methylation experiments published by Masunaga et al. (6), however, did not include positive control samples. An inclusion of appropriate controls would have corroborated the reliability of the results.
PTEN mutation, mono- or biallelic gene loss, and repression of expression are very frequent in human tumors (2). In thymomas and thymic carcinomas, however, PTEN mutations are either absent or very rare (8-14). So far, only a few studies have addressed PTEN protein expression in thymic epithelial tumors. Enkner et al. reported heterogenous PTEN protein expression by immunohistochemistry with anti-PTEN clone Y184, with no significant differences between type A and type B3 thymomas and thymic carcinomas (12). A lack of PTEN expression was noted in only one B3 thymoma and one thymic carcinoma. However, both tumors retained two normal PTEN alleles as assessed by fluorescence in-situ hybridization, and PTEN gene mutations were absent. Thus, the lack of PTEN expression in these two tumors may have been caused by an alternative mechanism. The results of Masunaga et al. suggest that the PTENP1 pseudogene is probably also not relevant for regulating PTEN protein abundancy in thymomas and thymic carcinomas (6).
Leisibach et al. (15) assessed PTEN expression only in the cytoplasm with a different anti-PTEN monoclonal antibody (clone D4.3). Whether a nuclear expression was also present as observed by Masunaga et al. (6) and Enkner et al. (12) is not reported. In their 21 analyzed thymoma and thymic carcinoma samples no significant difference in cytoplasmic PTEN protein expression was noted.
The lack of PTEN protein in non-neoplastic thymic epithelial cells and B1/B2 thymomas reported by Masunaga et al. (6) is intriguing. In mice, a PTEN knockout leads to embryonic lethality, and a heterozygous PTEN loss causes the emergence of tumors at various sites (16). A targeted heterozygous PTEN deletion in thymic epithelial cells of mice results in a smaller thymus with significantly disordered architecture and histology (17). Observations made by Masunaga et al., may lead us to infer that PTEN protein is not essential for epithelial cells in the human postnatal thymus, or that low PTEN protein levels that are not detected by immunohistochemistry are sufficient.
Analogous to the observation by Masunaga et al. (6) in normal thymic epithelia, PTEN protein is not expressed in normal muscle, liver, parathyroid, ovary and prostate glandular cells, although PTEN RNA is present (18). Therefore, in these tissues a regulatory mechanism of PTEN translational repression similar to that proposed by Masunaga et al. for non-neoplastic thymi might be at work. Apparently absent or low PTEN protein expression is not always tumorigenic, and in line with this conclusion a downregulation of PTEN protein has been implicated in enhanced tissue repair and regeneration (1,19).
The functional significance of the lack of PTEN protein in B1/B2 thymomas and of its expression in type A thymomas and thymic carcinomas observed by Masunaga et al. (6) is unclear. However, there seems to be no convincing evidence that PTEN is involved in the pathogenesis of thymic epithelial tumors. Therefore, a clinical impact of the work by Masunaga et al. can not be envisaged at present, although inhibition of the mTOR pathway which may be activated in PTEN repressed thymic epithelial cells is a therapeutic concept currently being validated in clinical trials (20-22).
A remarkable observation made by the Masunaga et al. (6) study is the proposed different regulation of PTEN RNA transcription/translation and protein stability between normal thymus and B1/B2 thymomas on the one hand and type A thymomas and thymic carcinomas on the other. Furthermore, of particular interest is the reported predominant nuclear localization of the PTEN protein in thymic epithelial tumors. Only in thymic carcinomas, and here only with one of the two antibodies employed, an additional weak cytoplasmic staining was observed. It remains to be determined whether one of the presumed nuclear PTEN functions, e.g., control of genomic stability, is of functional importance for the development or maintenance of the neoplastic phenotype in thymic epithelial tumors.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by Section Editor Dr. Qiangling Sun (The Central Laboratory in Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2018.10.02). LM serves as an unpaid editorial board member of Mediastinum from Feb 2018 to Jan 2020. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee YR, Chen M, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat Rev Mol Cell Biol 2018;19:547-62. [Crossref] [PubMed]
- Carracedo A, Alimonti A, Pandolfi PP. PTEN level in tumor suppression: how much is too little? Cancer Res 2011;71:629-33. [Crossref] [PubMed]
- Hobert JA, Eng C. PTEN hamartoma tumor syndrome: an overview. Genet Med 2009;11:687-94. [Crossref] [PubMed]
- Bassi C, Stambolic V. PTEN, here, there, everywhere. Cell Death Differ 2013;20:1595-6. [Crossref] [PubMed]
- Poliseno L, Salmena L, Zhang J, et al. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 2010;465:1033-8. [Crossref] [PubMed]
- Masunaga A, Omatsu M, Kunimura T, et al. Expression of PTEN and its pseudogene PTENP1, and promoter methylation of PTEN in non-tumourous thymus and thymic tumours. J Clin Pathol 2017;70:690-6. [Crossref] [PubMed]
- Wiencke JK, Zheng S, Jelluma N, et al. Methylation of the PTEN promoter defines low-grade gliomas and secondary glioblastoma. Neuro Oncol 2007;9:271-9. [Crossref] [PubMed]
- Petrini I, Meltzer PS, Kim IK, et al. A specific missense mutation in GTF2I occurs at high frequency in thymic epithelial tumors. Nat Genet 2014;46:844-9. [Crossref] [PubMed]
- Wang Y, Thomas A, Lau C, et al. Mutations of epigenetic regulatory genes are common in thymic carcinomas. Sci Rep 2014;4:7336. [Crossref] [PubMed]
- Shitara M, Okuda K, Suzuki A, et al. Genetic profiling of thymic carcinoma using targeted next-generation sequencing. Lung Cancer 2014;86:174-9. [Crossref] [PubMed]
- Moreira AL, Won HH, McMillan R, et al. Massively parallel sequencing identifies recurrent mutations in TP53 in thymic carcinoma associated with poor prognosis. J Thorac Oncol 2015;10:373-80. [Crossref] [PubMed]
- Enkner F, Pichlhöfer B, Zaharie AT, et al. Molecular Profiling of Thymoma and Thymic Carcinoma: Genetic Differences and Potential Novel Therapeutic Targets. Pathol Oncol Res 2017;23:551-64. [Crossref] [PubMed]
- Lee HS, Jang HJ, Shah R, et al. Genomic Analysis of Thymic Epithelial Tumors Identifies Novel Subtypes Associated with Distinct Clinical Features. Clin Cancer Res 2017;23:4855-64. [Crossref] [PubMed]
- Radovich M, Pickering CR, Felau I, et al. The Integrated Genomic Landscape of Thymic Epithelial Tumors. Cancer Cell 2018;33:244-58.e10. [Crossref] [PubMed]
- Leisibach P, Schneiter D, Soltermann A, et al. Prognostic value of immunohistochemical markers in malignant thymic epithelial tumors. J Thorac Dis 2016;8:2580-91. [Crossref] [PubMed]
- Di Cristofano A, Pesce B, Cordon-Cardo C, et al. Pten is essential for embryonic development and tumour suppression. Nat Genet 1998;19:348-55. [Crossref] [PubMed]
- Garfin PM, Nguyen T, Sage J. Loss of Pten Disrupts the Thymic Epithelium and Alters Thymic Function. PLoS One 2016;11:e0149430 [Crossref] [PubMed]
- Uhlen M, Fagerberg L, Hallstrom BM, et al. Proteomics. Tissue-based map of the human proteome. Science 2015;347:1260419 [Crossref] [PubMed]
- Naguib A, Trotman LC. PTEN plasticity: how the taming of a lethal gene can go too far. Trends Cell Biol 2013;23:374-9. [Crossref] [PubMed]
- Dillon LM, Miller TW. Therapeutic targeting of cancers with loss of PTEN function. Curr Drug Targets 2014;15:65-79. [Crossref] [PubMed]
- Owen DH, Otterson GA. Everolimus in thymic epithelial tumors: practical considerations. Mediastinum 2018;2.
- Zucali PA, De Pas T, Palmieri G, et al. Phase II Study of Everolimus in Patients With Thymoma and Thymic Carcinoma Previously Treated With Cisplatin-Based Chemotherapy. J Clin Oncol 2018;36:342-9. [Crossref] [PubMed]
Cite this article as: Oberndorfer F, Müllauer L. PTEN tumor suppressor expression in thymic epithelial tumors. Mediastinum 2018;2:59.


