
Fully-circumferential tracheal replacement: when and how?
Extensive tracheal resections are mainly considered in the treatment of extended malignant lesions. These are respectively: (I) for proximal tumors, monobloc laryngotracheal resection followed by construction of an anterior mediastinal tracheostomy (associated, when appropriate, with trans-hiatal esophagectomy and esophageal substitution by gastroplasty or coloplasty) (1); (II) segmental resection of the trachea over 50% of its length in adults and over 30% in children; (III) carinal resection, isolated or associated with pneumonectomy, when a greater than 4 cm airway gap makes end-to-end anastomosis impossible. In the latter two situations, the use of a tracheal substitute for fully-circumferential tracheal replacement (FTR) is mandatory. Occasionally, the tracheal substitute may be useful to treat a large congenital/acquired benign stenosis or malacia, or a dehiscence after tracheal or cricotracheal resection reconstruction by primary anastomosis (2).
The development of such a substitute has been the subject of an impressive number of experimental studies. However, they have only led to rare clinical applications (3). In this respect, it should be noted that is often confusion in several published reviews between patch tracheal reconstruction [i.e., conservation of the native posterior tracheal wall, such as performed by Jacobs and coworkers (4), or Delaere and coworkers (5)], and fully-circumferential reconstructions, which pose the most difficult technical issues (3).
Aside from desired biomechanical properties already detailed by Belsey (6), another major requirement for tracheal substitutes should be the absence of additional immunosuppressive therapy, strongly contraindicated in cancer patients. In fact, few substitutes meet this requirement: prostheses, aortic allografts, bioengineered tracheal matrices, and finally, autologous tissues. In this field, in addition to a previous report (7), Mercier and coworkers now report their cumulative experience of the autologous FTR by means of a cartilaginous-armed forearm free flap (8).
Prior to this recent study, the former reported case series were FTRs using Marlex mesh or silicone prostheses (9-11), but major complications were observed, such as chronic infections and especially arterial erosions leading to the abandonment of these techniques at the end of the 1980s.
Later on, we launched a research program of tracheal replacement with silicone-stented aortic allografts for unresectable tumors. From March 2005 to September 2007, six patients were included in the study (12,13). Despite the fact that the FTR involved the carinal region in four cases, there was no in-hospital mortality and the present editorial is an opportunity for us to update patients’ data, which are summarized in Table 1. Among the six patients, three died at 26, 45, and 77 months, and three are still alive at 149+, 136+ and 133+ months, respectively (two of them with no evidence of disease). However, although this extensive resection without additional radiation therapy achieved local control in 5/6 patients, it did not avoid the occurrence of metastatic disease in the long term. Thereafter, Martinod and coworkers reported their experience of tracheal and bronchial reconstruction using a similar procedure. In their group of 13 patients, only one underwent an FTR after a carinal right pneumonectomy and, unfortunately, died of a cerebrovascular event in the postoperative period. There were five additional, albeit non-circumferential, tracheal reconstructions in the cervical area. These latter patients were found to have satisfactory results and the possibility of stent removal in three of them (14).
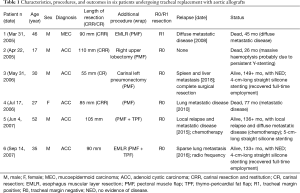
Full table
Numerous problems have affected the field of bioengineered trachea (15,16). Consequently, only two well-documented cases of bioengineered tracheal matrices used for FTR, both reported by Elliott and coworkers, have been published (17,18). The former patient showed occurrence of a severe shrinking of the substitute over several years (19), which casts doubts on cartilage regeneration into the tracheal matrix (20), and the latter died of respiratory distress in the postoperative period, precluding any long-term conclusions (18).
In the current study, starting in September 2006, the experience of Mercier and coworkers constitutes an important contribution to the field of FTR. In their article, they provide a comprehensive description of the cartilaginous-armed forearm free flap used to construct the tracheal neo conduit. This construct was implanted in 16 patients, after extended tracheal resection (mainly for tumors) in ten, and for elongation of the tracheal stump to create a cervical tracheostomy after tracheolaryngectomy in six (8). This relevant work deserves some comments. First, the authors might have made reference to the seminal article from Olias and coworkers (21) describing for the first time [2005] the use of the cartilage-reinforced radial forearm free flap in reconstruction of a fully-circumferential tracheal defect of the trachea. Interestingly in the Olias study compared to the current study, an epithelial lining was achieved by using buccal mucosa, to overcome the problem of mucus plugging as a consequence of poor clearance properties of the squamous cell epithelium lining of the neo conduit. Second, as outlined by the authors of the current study, the use of autologous costal cartilages to construct the neo tracheal rings is a source of problems due to cartilage calcification over the years leading to possible cartilage fracture reducing the airway lumen; or even constituting a real contraindication to the procedure. Third, despite the reliability of the forearm free flap, there is a potential risk of ischemia/necrosis, a life-threatening complication occurring in one of their patients, in which three consecutive free-flap procedures were finally required. Fourth, in the authors’ series, of six patients receiving an autologous tracheal replacement to create a cervical tracheostomy after tracheolaryngectomy, one sustained innominate artery rupture and died six months after surgery. Therefore, the superiority of this technique of tracheal elongation is not demonstrated, compared to the anterior mediastinal tracheostomy with construction of the mediastinal stoma by means of a pectoralis major myocutaneous island flap, which has emerged as the gold standard after extended laryngotracheal resection (22). Finally, for Mercier and coworkers, the main indication of autologous FTR was locally advanced adenoid cystic carcinoma (ACC) of the trachea (ten cases). Given that the disease is characterized by extensive sub-mucosal invasion, it would have been interesting to specify the tracheal margins status (R0 vs. R1 resection), as stated in the authors’ previous article (in which 4/5 patients having undergone resection for ACC were found to have positive tracheal margins) (7). A high frequency of positive margins could cast doubts as to the true curative nature of such extensive surgery and its potential advantage. In fact, according to the 100% efficacy of the chemoradiation therapy, based on carboplatin paclitaxel regimen in locally advanced tracheal and laryngeal ACC reported by Allen and coworkers (23), and then Misiukiewicz and coworkers (24), this approach might have been discussed as a valuable alternative therapy (25). Thus, after discussion through electronic communication with Aaron Allen at the end of 2007 (who confirmed to us the long-term satisfactory results of the chemoradiation therapy in three patients), our group decided not to perform FTR in further patients suffering from extended tracheal ACC.
We have conducted an in-depth study of the available literature in the setting of fully-circumferential tracheal/carinal replacement with different substitutes. We have excluded the historical series of prosthetic FTRs reported up to 1990, characterized by a high level of morbidity and mortality (9-11), and we have discarded a recently retracted article (16). We have also excluded the case series of patch tracheal reconstructions (4,5), and the cases of tracheal stump elongation after tracheolaryngectomy by means of free flap procedures, which pose other surgical issues (22).
By including the ten cases reported by Mercier and coworkers (8), we have finally identified 31 well-documented cases of FTR for both malignant (n=21) and benign (n=10) pathology. After FTR, the overall in-hospital mortality rate was 16% (5/31). The rate was zero (0/10) in the cervical area, 14% (2/14) in the cervicomediastinal or mediastinal area, and 43% (3/7) when the FTR involved the carina.
According to this study, one can conclude that cervical FTRs are valuable indications. Mediastinal FTRs might be discussed on a case-by-case basis. In contrast, FTRs associated with carinal reconstruction should be discouraged. In the case of ACC, risks of FTR (overall in-hospital mortality rate of 25%, 4/16 patients) balanced with benefits advocate for definitive chemoradiotherapy, which has been demonstrated as effective in tracheal/carinal tumor location (23).
In practice, both aortic allografts (n=10) and composite grafts based on the forearm free flap (n=14) have been widely used for FTR, with quite similar results in the postoperative period [in-hospital mortality rate of 20% (2/10 patients) vs. 21% (3/14 patients)]. The aortic allograft has several advantages: it is readily available in tissue banks, microbiologically safe, and its availability is particularly useful in an emergency setting (2). From a practical point of view, a complete graft wrap with bulky and well-vascularized flaps is required to promote neoangiogenesis and avoid microfistulization (13,14). Despite the fact that cartilage regeneration into the aortic graft remains controversial, it has been demonstrated that some grafts were stiff enough to allow stent removal in the mid/long term (14). On the other hand, composite grafts based on the forearm free flap achieve well-vascularized and rigid tracheal conduits from the onset but are more complex and time-consuming procedures. Furthermore, a free-flap ischemia can occur, requiring emergent salvage surgery for reperfusion (or repeated free-flap procedure in the case of impossible reperfusion). Last, delayed cartilage fracture and consecutive airway collapse can also occur requiring tracheal stenting, or permanent tracheotomy (8).
Finally, in the setting of tracheal allotransplantation after heterotopic revascularization in the forearm, Delaere and coworkers propose an ingenious concept for fully-circumferential tracheal reconstruction. Cartilaginous portions of trachea and left main bronchus retrieved from a cadaveric allograft are sutured together to obtain a patch with extended width. After revascularization and mucosal regeneration (pretransplant period), the patch is tubed and used for the free-flap fully-circumferential tracheal repair. However, the need for immunosuppressive therapy during the pretransplant period constitute a potential limitation of this technique, which has not been performed to date (5).
Acknowledgments
The author thanks Professor Eric Kipnis for editorial assistance, and friendship.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by Section Editor Dr. Zhuoqi Jia (Thoracic Department, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2018.12.02). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Conti M, Benhamed L, Mortuaire G, et al. Indications and results of anterior mediastinal tracheostomy for malignancies. Ann Thorac Surg 2010;89:1588-95. [Crossref] [PubMed]
- Zanetta A, Cuestas G, Rodríguez H, et al. Laryngotracheal reconstruction with cryopreserved aortic allograft as a salvage technique when cricotracheal resection complications occur in paediatrics. Acta Otorrinolaringol Esp 2014;65:191-3. [Crossref] [PubMed]
- Rich JT, Gullane PJ. Current concepts in tracheal reconstruction. Curr Opin Otolaryngol Head Neck Surg 2012;20:246-53. [Crossref] [PubMed]
- Jacobs JP, Elliott MJ, Haw MP, et al. Pediatric homograft reconstruction: a novel approach to complex tracheal stenosis in children. J Thorac Cardiovasc Surg 1996;112:1549-58; discussion 1559-60. [Crossref] [PubMed]
- Delaere P, Lerut T, Van Raemdonck D. Tracheal Transplantation: State of the Art and Key Role of Blood Supply in Its Success. Thorac Surg Clin 2018;28:337-45. [Crossref] [PubMed]
- Belsey R. Resection and reconstruction of the intrathoracic trachea. Br J Surg 1950;38:200-5. [Crossref] [PubMed]
- Fabre D, Kolb F, Fadel E, et al. Successful tracheal replacement in humans using autologous tissues: an 8-year experience. Ann Thorac Surg 2013;96:1146-55. [Crossref] [PubMed]
- Mercier O, Kolb F, Dartevelle PG. Autologous Tracheal Replacement: Surgical Technique and Outcomes. Thorac Surg Clin 2018;28:347-55. [Crossref] [PubMed]
- Pearson FG, Thompson DW, Weissberg D, et al. Adenoid cystic carcinoma of the trachea. Experience with 16 patients managed by tracheal resection. Ann Thorac Surg 1974;18:16-29. [Crossref] [PubMed]
- Neville WE, Bolanowski PJ, Kotia GG. Clinical experience with the silicone tracheal prosthesis. J Thorac Cardiovasc Surg 1990;99:604-12; discussion 612-3. [PubMed]
- Toomes H, Mickisch G, Vogt-Moykopf I. Experiences with prosthetic reconstruction of the trachea and bifurcation. Thorax 1985;40:32-7. [Crossref] [PubMed]
- Wurtz A, Porte H, Conti M, et al. Tracheal replacement with aortic allografts. N Engl J Med 2006;355:1938-40. [Crossref] [PubMed]
- Wurtz A, Porte H, Conti M, et al. Surgical technique and results of tracheal and carinal replacement with aortic allografts for salivary gland-type carcinoma. J Thorac Cardiovasc Surg 2010;140:387-93.e2. [Crossref] [PubMed]
- Martinod E, Chouahnia K, Radu DM, et al. Feasability of Bioengineered Tracheal and Bronchial Reconstruction Using Stented Aortic Matrices. JAMA 2018;319:2212-22. [Crossref] [PubMed]
- Wurtz A, Kipnis E. Tissue-engineered airway in the clinical setting: a call for information disclosure. Clin Pharmacol Ther 2012;91:973. [Crossref] [PubMed]
- . The Lancet. The final verdict on Paolo Macchiarini: guilty of misconduct. Lancet 2018;392:2. [Crossref]
- Elliott MJ, De Coppi P, Speggiorin S, et al. Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet 2012;380:994-1000. [Crossref] [PubMed]
- Elliott MJ, Butler CR, Varanou-Jenkins A, et al. Tracheal Replacement Therapy with a Stem Cell-Seeded Graft: Lessons from Compassionate Use Application of a GMP-Compliant Tissue-Engineered Medicine. Stem Cells Transl Med 2017;6:1458-64. [Crossref] [PubMed]
- Hamilton NJ, Kanani M, Roebuck DJ, et al. Tissue-engineered tracheal replacement in a child: A 4-year follow-up study. Am J Transplant 2015;15:2750-7. [Crossref] [PubMed]
- Wurtz A, Hysi I, Kipnis E, et al. Recent Advances in Circumferential Tracheal Replacement and Transplantation. Am J Transplant 2016;16:1334-5. [Crossref] [PubMed]
- Olias J, Millán G, da Costa D. Circumferential tracheal reconstruction for the functional treatment of airway compromise. Laryngoscope 2005;115:159-61. [Crossref] [PubMed]
- Wurtz A, De Wolf J. Anterior Mediastinal Tracheostomy: Past, Present, and Future. Thorac Surg Clin 2018;28:277-84. [Crossref] [PubMed]
- Allen AM, Rabin MS, Reilly JJ, et al. Unresectable adenoid cystic carcinoma of the trachea treated with chemoradiation. J Clin Oncol 2007;25:5521-3. [Crossref] [PubMed]
- Misiukiewicz KJ, Camille N, Tishler R, et al. Organ preservation for adenoid cystic carcinoma of the larynx. Oncologist 2013;18:579-83. [Crossref] [PubMed]
- Wurtz A. Circumferential tracheal replacement: do the benefits warrant the risks? Ann Thorac Surg 2014;97:1480. [Crossref] [PubMed]
Cite this article as: Wurtz A. Fully-circumferential tracheal replacement: when and how? Mediastinum 2019;3:1.


