
胸腺恶性肿瘤内镜下淋巴结清扫的策略:经侧胸肋间入路和剑突下入路方法的探讨
引言
目前针对胸腺恶性肿瘤淋巴结清扫的意义尚不明确。据统计,约25%~26.8%的胸腺癌以及13.3%的胸腺瘤出现了淋巴结转移,同时发现淋巴结是否转移是评估胸腺恶性肿瘤患者预后的重要因素[1-6]。
近年来,内镜手术是外科治疗胸腺恶性肿瘤的重要方式之一。针对胸腺恶性肿瘤的手术入路主要有三种:经颈部入路,经侧胸入路以及经剑突下入路。每一种手术入路对淋巴结清扫的范围均存在差异。本综述旨在讨论针对胸腺恶性肿瘤行剑突下或侧胸入路的淋巴结清扫,每种入路方法下可能发生淋巴结转移的范围。
胸腺恶性肿瘤的内镜手术策略
目前临床上使用的微创全胸腺切除手术方式主要有三种,经颈部入路的手术、经侧胸肋间入路胸腔镜手术、经剑突下入路胸腔镜手术。经颈部入路的手术没有广泛使用,原因在于对上纵隔胸腺的暴露差以及操作困难。经侧胸入路手术是目前使用较为普遍的手术方式,经左胸或右胸均可做为常规的手术入路。其优势在于微创,但对颈部胸腺暴露差,对肋间神经也有损伤,对侧膈神经的显露差。我们曾经在2012年报道了单孔剑突下全胸腺切除术,在2015年报道了剑突下机器人辅助全胸腺切除术[6-8],总结了剑突下入路手术的优势在于良好的颈部胸腺和双侧膈神经的暴露。
针对胸腺恶性肿瘤的淋巴结清扫范围
大多数胸外科医师可能不会在胸腺恶性肿瘤手术中进行淋巴结取样或清扫。但对胸腺恶性肿瘤行淋巴结采样或清扫能够获得肿瘤准确的病理分期。针对胸腺恶性肿瘤术后辅助治疗的效果尚不清晰,淋巴结采样或清扫能够为术后辅助治疗提供病理学依据,从而最大程度改善患者的预后。国际胸腺肿瘤协作组(International Thymic malignant Interesting Group, ITMIG)病理分期系统将纵隔淋巴结分为两类,一类为前纵隔或环胸腺淋巴结;另一类为纵隔深部淋巴结[9]。前纵隔淋巴结如有转移被命名为N1,其对应的病理分期为Iva期;纵隔深部或颈部淋巴结转移被命名为N2,其对应的病理分期为IVb期。前纵隔淋巴结的上界为舌骨水平,在内镜下对舌骨水平淋巴结的清扫则较为困难。针对胸腺恶性肿瘤的淋巴结清扫范围上达甲状腺,两侧达双侧膈神经,下达剑突和膈肌水平。当以上区域内的所有脂肪连同胸腺一并被切除时,大多数的前纵隔淋巴结也可以一并切除。此类手术方法与重症肌无力行胸腺扩大切除术极为相似。在开胸手术中,颈部和纵隔深部淋巴结也未进行常规清扫和采样,但有文献报道称纵隔深部的右侧气管旁淋巴结也经常发生转移[3]。因此,对右侧气管旁淋巴结的采样或者清扫是有必要的。
经侧胸入路内镜下淋巴结清扫范围
经侧胸入路的内镜下淋巴结清扫范围从同侧膈神经跨越胸腺至对侧前上纵隔区域。此入路的缺点是无法完全暴露对侧膈神经,因此也无法完全清扫所有前纵隔淋巴结。如想要根治性切除所有前纵隔区域内的淋巴结,则需选择双侧侧胸入路。同时,经侧胸入路无法获得从无名静脉面向颈部的视野。虽然经侧胸入路达到对侧前纵隔淋巴结的完全清扫较为困难,但对纵隔深部淋巴结的处理则较为方便。经右胸入路更易清扫气管旁淋巴结,而经左胸入路更易清扫主动脉旁和主动脉下淋巴结,采用侧卧位更易清扫隆突下淋巴结。图1展示了患者通过仰卧位经侧胸入路行气管旁淋巴结清扫,术中发现患者气管旁淋巴结肿大明显并伴有胸腺增生。术后病理显示胸腺增生且未见淋巴结转移。因此,侧胸入路能够更方便地清扫气管旁淋巴结 (图1)。

经剑突入路内镜下淋巴结清扫范围
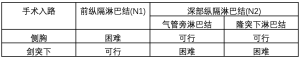
剑突下入路的优势则是将内镜镜头置于身体中线,能够更好地暴露颈部和双侧膈神经。经剑突下入路行前纵隔淋巴结清扫能够达到和胸骨正中开放手术相似的效果,但其对纵隔深部淋巴结的清扫则较为困难。经剑突下入路也能完成气管旁淋巴结清扫,但对隆突下淋巴结的清扫则较为困难。图2展示了机器人辅助剑突下全胸腺切除(图2)。患者为89岁老年男性,术前诊断为胸腺恶性肿瘤,术中探查发现左肺与胸壁致密粘连。经充分暴露两侧膈神经后,发现肿瘤明显侵犯左无名静脉和右侧部分肺组织。因此,手术选择全胸腺切除联合左侧无名静脉和右肺切除。术中行人工血管植入,前纵隔淋巴结清扫以及右侧气管旁淋巴结采样。术中共26枚淋巴结被切除,术后病理检查显示未见转移淋巴结。表1总结了经剑突下入路和侧胸入路内镜下淋巴结清扫的数据 (表1)。

Full table
评论
虽然经右胸入路完全清扫前纵隔淋巴结较为困难,但对气管旁淋巴结的清扫则较为方便。经左胸入路完全清扫前纵隔淋巴结也较为困难,但对主动脉旁和主动脉下淋巴结的清扫则较为方便。因主动脉旁和主动脉下淋巴结的转移极为罕见,不建议经左胸入路行淋巴结清扫。经双侧入路能够完成前纵隔淋巴结、气管旁淋巴结、主动脉旁淋巴结以及隆突下淋巴结的清扫。如采用侧卧位,无论经右胸或左胸入路,均能够行隆突下淋巴结清扫。而剑突下入路能够完成前纵隔和气管旁淋巴结的清扫,对隆突下淋巴结的清扫则较为困难。
如果主要目的是清扫前纵隔淋巴结,经剑突下或双侧入路均能达到理想的清扫效果。经剑突下内镜淋巴结清扫能够达到和胸骨正中切口开放手术相似的效果。因此,针对胸腺恶性肿瘤内镜下淋巴结清扫更倾向于使用侧胸入路。目前,胸外科医生不常选择剑突下入路的原因可能是对此项技术熟悉度的欠缺。如果不局限于单孔手术,或使用机器人辅助手术,通过联合侧胸和剑突下手术,对于熟悉剑突下手术的技术要点并不困难。本文推荐使用剑突下入路行内镜下淋巴结清扫,尤其是针对前纵隔淋巴结,其能达到与开放手术相当的清扫效果。
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors Mirella Marino and Brett W. Carter for the series “Dedicated to the 9th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2018)” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2019.03.02). The series “Dedicated to the 9th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2018)” was commissioned by the editorial office without any funding or sponsorship. TS serves as an unpaid editorial board member of Mediastinum from Oct 2018 to Sep 2020. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. [Crossref] [PubMed]
- Kondo K, Monden Y. Lymphogenous and hematogenous metastasis of thymic epithelial tumors. Ann Thorac Surg 2003;76:1859-64; discussion 1864-5.
- Hwang Y, Park IK, Park S, et al. Lymph Node Dissection in Thymic Malignancies: Implication of the ITMIG Lymph Node Map, TNM Stage Classification, and Recommendations. J Thorac Oncol 2016;11:108-14. [Crossref] [PubMed]
- Weksler B, Pennathur A, Sullivan JL, et al. Resection of thymoma should include nodal sampling. J Thorac Cardiovasc Surg 2015;149:737-42. [Crossref] [PubMed]
- Gu Z, Wei Y, Fu J, et al. Lymph node metastases in thymic malignancies: a Chinese Alliance for Research in Thymomas retrospective database analysis. Interact Cardiovasc Thorac Surg 2017;25:455-61. [Crossref] [PubMed]
- Suda T, Sugimura H, Tochii D, et al. Single-port thymectomy through an infrasternal approach. Ann Thorac Surg 2012;93:334-6. [Crossref] [PubMed]
- Suda T, Hachimaru A, Tochii D, et al. Video-assisted thoracoscopic thymectomy versus subxiphoid single-port thymectomy: initial results†. Eur J Cardiothorac Surg 2016;49:i54-8. [PubMed]
- Suda T, Tochii D, Tochii S, et al. Trans-subxiphoid robotic thymectomy. Interact Cardiovasc Thorac Surg 2015;20:669-71. [Crossref] [PubMed]
- Carter BW, Benveniste MF, Madan R, et al. IASLC/ITMIG Staging System and Lymph Node Map for Thymic Epithelial Neoplasms. Radiographics 2017;37:758-76. [Crossref] [PubMed]
- Suda T. Paratracheal lymph node dissection via the lateral thoracic intercostal approach with the patient in the dorsal position. Asvide 2019;6:086. Available online: http://www.asvide.com/article/view/30851
- Suda T. Lymph node dissection using robot-assisted thymectomy via the subxiphoid approach. Asvide 2019;6:087. Available online: http://www.asvide.com/article/view/30852

房渝
现为重医大附一院普胸外科主治医师、讲师、博士。中华医学会胸心血管外科分会会员,重庆市医学会胸心外科学分会会员,重庆市医药技术协会肺癌专委会委员,重庆市医药技术协会食管癌专委会委员,中国临床肿瘤学会会员,中国胸壁联盟资深手术专家。美国北卡罗莱纳中央大学(NCCU)访问学者。承担国家自然科学基金1项,发表SCI论文17篇。为American Journal of Clinical and Experimental Medicine 杂志Section Editor,Journal of Cardiovascular Surgery杂志Reviewer。(更新时间:2021/7/26)
(本译文仅供学术交流,实际内容请以英文原文为准。)
Cite this article as: Suda T. Endoscopic lymph node dissection for thymic malignancies: lateral thoracic intercostal and subxiphoid approaches. Mediastinum 2019;3:10.


