
Immune checkpoint inhibitors for treatment of thymic epithelial tumors: how to maximize benefit and optimize risk?
Introduction
Thymic epithelial tumors (TETs) are neoplasms arising from epithelial cells of the thymus. Although relatively rare, with an incidence of approximately 1.5 cases/million, TETs are the most common malignancy of the anterior mediastinum in adults (1). Histologic classification of TETs is based on the World Health Organization (WHO) system, which was updated most recently in 2015 (2). TETs, primarily thymoma, are accompanied by a variety of autoimmune disorders including myasthenia gravis, pure red cell aplasia and Good’s syndrome (3).
Surgery is usually the treatment of choice and the only curative option if the disease is localized. Platinum-based chemotherapy is used for treatment of locally-advanced or metastatic disease (4). However, treatment options for relapsed or refractory disease are limited (5). Targeted therapies, such as epidermal growth factor receptor inhibitors (gefitinib, erlotinib with bevacizumab, cetuximab), angiogenesis inhibitors (sunitinib and aflibercept), c-KIT pathway inhibitors (imatinib), histone deacetylase inhibitors (belinostat), octreotide, and Src inhibitors (saracatinib), among others, have been evaluated in relapsed and refractory TETs, but have shown modest and short-lived responses (6-9).
TETs have the lowest tumor mutation burden (TMB) among all adult cancers (10,11), which limits identification of new drug targets. Hence, novel approaches need to be considered to overcome the challenges associated with drug development for relapsed or refractory TETs. Activation of antitumor immunity is a promising option. However, the uniqueness of thymic physiology influences the risks and potential benefits of immunotherapy for TETs.
Immunotherapy for treatment of cancer
Development of cancer is fundamentally related to defects in immune surveillance and the inability of the immune system to eliminate neoplastic cells in the early stages of tumor formation (12). Multiple immunotherapeutic modalities have been developed to overcome immune paresis and activate antitumor immunity including adoptive cell therapy (chimeric antigen receptor T cell therapy, T cell receptor therapy and tumor infiltrating lymphocyte therapy), cancer vaccines and immune checkpoint inhibitors (ICIs) [e.g., targeting programmed death-1 (PD-1) or its ligand (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)] (13). Amongst these options, ICIs are some of the most widely used forms of immunotherapy in clinical practice and are associated with objective responses and durable benefit in a subset of patients with a variety of cancers including carcinogen-induced cancers, cancers driven by viral infections such as Merkel cell carcinoma, microsatellite instability-high (MSI-H) tumors, desmoplastic melanoma, and Hodgkin’s disease (14-16).
Efforts are underway to identify biomarkers that predict for response to ICIs (17). Tumor cell PD-L1 expression and TMB are established biomarkers of response. PD-L1 activates the inhibitory signaling pathway on anti-tumor T cells by binding to PD-1, and its expression level influences the activity of anti-PD-1 and anti-PD-L1 ICIs. PD-L1 is the most commonly used predictive biomarker for anti-PD-1/PD-L1 therapies (18,19).
The adaptive immune system recognizes tumor cells as foreign (non-self) via antigen recognition; hence, the quantity and quality of tumor antigens has a direct effect on anti-tumor response. TMB, which reflects the number of non-synonymous single nucleotide variants (nsSNVs) in a tumor affects the odds of generating antigens that can trigger an anti-tumor immune response and thereby influence the ability of ICIs to generate clinical responses in patients. Higher TMB implies more neoantigens in tumors, which can facilitate immune recognition and elicit T-cell responses. Several clinical trials in non-small cell lung cancer (NSCLC) have validated this correlation (20,21). Higher TMB is also associated with improved survival in patients receiving immunotherapy independent of cancer type (22).
In addition, robust anti-tumor immune response requires pre-existing presence of tumor-infiltrating lymphocytes (TILs). Therefore, the density of TILs also correlates with patients’ response to immunotherapy (23).
Despite these advances, not all patients destined to benefit from immunotherapy can be identified using tumor cell PD-L1 expression and TMB. Hence, there are ongoing attempts to develop a composite biomarker that can help identify the immunotherapeutic modality of choice for a given patient (24).
Activation of anti-cancer immunity also increases the risk of developing immune-related adverse events (irAEs) (25). The majority of irAEs in non-TET patients are of low to moderate grade and are manageable with established treatment protocols (26,27). However, some patients can experience life-threatening toxicity and efforts are underway to identify biomarkers that can predict the risk for development of irAEs (28).
Immunotherapy for TETs
PD1/PD-L1 expression and TMB in TETs
PD-L1 expression in TETs has been widely evaluated. Although there are variations due to the assays used and measurement cutoffs, PD-L1 is commonly expressed in TETs: 23–92% of tumor cells in thymoma and 36–100% of tumor cells in thymic carcinoma express PD-L1 (29-32). These results provide a rationale for using PD-1/PD-L1 inhibitors to treat TETs.
However, TETs also have the lowest TMB among all adult cancers (10). Although lower TMB is usually associated with a lower response rate to ICIs, the initial experience with ICIs in TETs (described below) demonstrates a relatively high response rate despite a low TMB (33-35). The discordance between low TMB and a higher-than-expected response rate in TETs is likely related to the unique biology of the thymus and its role in T-cell development.
Implications of thymic physiology on immunotherapy for TETs
The thymus plays a crucial role in the development of immune tolerance. T cell progenitors undergo maturation in the thymus through interactions with cortical and medullary thymic epithelial cells. This process requires normal thymic architecture (cortex and medulla), and expression of major histocompatibility complex (MHC) class II and the autoimmune regulator (AIRE) genes (36).
As T cell progenitors pass through the thymic cortex and corticomedullary junction, a series of phenotypic modifications occur, which eventually results in the generation of a functioning T cell receptor. Immature T cells that do not react with MHC class II are purged whereas those that do are said to have undergone “positive selection” and enter the thymic medulla. The medulla contains both medullary thymic epithelial cells (mTECs) and dendritic cells. mTECs express various tissue-specific self-antigens (TSAs), an activity which is tightly controlled by the AIRE gene (37). mTECs expressing AIRE undergo rapid turnover that eventually results in apoptosis, releasing TSAs to thymic dendritic cells, which in turn present TSAs to developing T cell progenitors. T cells that react against TSAs (autoreactive T cells) undergo apoptosis. This process of “negative selection” results in the development of immune tolerance.
TETs lack normal thymic architecture and have abnormal thymic epithelial cells, downregulated MHCII and absence of AIRE expression. As a result, thymocyte development is dysfunctional and autoreactive T cells are released into the circulation due to absence of negative selection, resulting in an increased predisposition towards development of autoimmune disease (38,39). Additionally, autoreactive T cells can recognize self-antigens expressed on TET tumor cells and release interferon-gamma (IFN-γ), which can upregulate PD-L1 expression in TET tumor cells (40). These findings provide an explanation for high tumor cell PD-L1 expression in TETs.
Taken together, high tumor cell PD-L1 expression provides a rationale for using ICIs for treatment of TETs, whereas presence of autoreactive T cells in the circulation places TET patients at an increased risk for developing irAEs upon receiving immunotherapy when compared with patients with other malignancies.
Clinical trials evaluating immunotherapy for TETs
Immune checkpoint inhibitors
The anti-PD-1 antibody, pembrolizumab, and the anti-PD-L1 antibody, avelumab, have been evaluated in patients with recurrent TETs (33,34,41).
Giaccone et al. conducted a single-arm, phase 2 study of pembrolizumab in patients with recurrent thymic carcinoma. Patients with prior history of autoimmune disease were excluded from this trial. Among 40 evaluable patients, an overall response rate (ORR) of 22.5% was observed. The median duration of response was 22.4 months. Median progression-free survival (mPFS) was 4.2 months and median overall survival (OS) was 24.9 months. One-year PFS and OS were 29% and 71%, respectively. High PD-L1 expression was associated with longer survival (median PFS 24 vs. 2.9 months; median OS not reached vs. 15.5 months) (34).
Cho et al. evaluated pembrolizumab in 26 patients with recurrent thymic carcinoma and 7 patients with recurrent thymoma. Patients with active autoimmune disease requiring systemic treatment or a history of severe autoimmune disease were ineligible. The ORR was 19.2% in patients with thymic carcinoma and 28.6% in patients with thymoma. Tumors with high PD-L1 expression were more likely to respond to treatment. The median duration of response was not reached in patients with thymoma and was 9.7 months in patients with thymoma carcinoma. Median PFS was 6.1 months in both groups. Median OS was 14.5 months for thymic carcinoma and not reached in patients with thymoma (33).
Rajan et al. evaluated avelumab, in 8 TET patients (7 thymoma and 1 thymic carcinoma) with no history of autoimmune disease. Four of 7 patients with thymoma had an objective response including a confirmed partial response in 2 (29%) patients. Significant tumor shrinkage was observed after one dose of avelumab in three patients (41).
These trials demonstrate the clinical activity of PD-1/PD-L1 inhibitors in patients with recurrent TETs (Table 1). High PD-L1 expression appears to be associated with a greater likelihood of response and a subset of patients achieve durable responses.

Full table
Cancer vaccines and other immunomodulatory strategies
Since TETs have a low TMB, few neoantigens have been identified, which has restricted development of cancer vaccines and adoptive T cell therapies. Several innovative neoantigen identification strategies are under investigation in refractory solid tumors incorporating high variant allele frequency, HLA-binding affinity and patients’ hotspot mutation (42).
These efforts have resulted in the discovery of neoantigens in a patient with relapsed thymoma. The patient received a personalized dendritic cell vaccine targeting a somatic mutation of the CDC73 gene and achieved a durable complete response. Evaluation of peripheral blood mononuclear cells showed a strong immunologic response to the epitope of mutated CDC73 protein (42).
Wilms’ tumor-1 (WT-1) has also been identified as a neoantigen in TETs and a WT1 peptide-based vaccine immunotherapy has undergone evaluation in patients with advanced TETs. Disease stabilization was seen in most vaccinated patients (75%) accompanied by induction of a WT1-specific immune response (43,44).
In addition to directly targeting antigens on tumor cells, radiation therapy has also been used to generate an immune response against TETs by harnessing post-treatment abscopal effects (45).
Immunotherapy increases risk for autoimmune toxicity in TET patients
Since TETs, especially thymomas, are associated with defective immune tolerance, these tumors are associated with a wide spectrum of paraneoplastic autoimmune disorders (3,46). The most common autoimmune condition is myasthenia gravis, which is usually caused by antibodies to the acetylcholine receptor at the neuromuscular junction. The predisposition to paraneoplastic autoimmunity places TET patients at high risk for developing severe autoimmune toxicity upon treatment with immunotherapy when compared with patients with other malignancies.
Among the three published trials evaluating ICIs in TETs, 15–62.5% of patients developed irAEs (Table 2) (33,34,41). Although the incidence of irAEs is higher in association with thymoma, patients with thymic carcinoma are also at greater risk of developing immune-related toxicity.
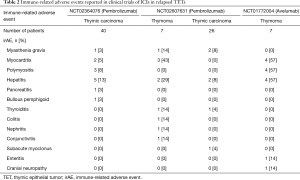
Full table
Effect on the neuromuscular junction and skeletal muscle
Immune-related toxicity can affect virtually any organ system (25). However, the incidence of irAEs involving the neuromuscular junction or skeletal muscle is strikingly high in TET patients receiving immunotherapy.
Myasthenia gravis has been reported as an irAE in 3–14% of TET patients treated with pembrolizumab (33,34). Myasthenia gravis also developed many months after a thymoma patient received WT-1-peptide based vaccination (43). In contrast, myasthenia gravis as a complication of immunotherapy has been reported rarely in patients with other cancers.
Myositis was observed in 8% of patients with thymic carcinoma treated with pembrolizumab and more than half of thymoma patients enrolled on a dose-escalation trial of avelumab (34,41). The incidence of myositis is much lower in non-TET patients treated with immunotherapy. This is illustrated by a retrospective analysis of patients with melanoma treated with pembrolizumab or nivolumab, among whom 1.4% developed myositis (47).
The propensity to develop myositis and neuromuscular adverse events appears to be related to the presence of a TET rather than the type of immunotherapy. Interestingly, myasthenia gravis and polymyositis have also been observed in patients with thymoma after treatment with non-immunotherapy drugs, albeit those with immunomodulatory properties, such as the insulin-like growth factor receptor-1 inhibitor, cixutumumab and the multi-kinase inhibitor, sunitinib (7,48,49).
Effect on the myocardium
Myocarditis is a rare but serious autoimmune condition associated with TETs (<1%) (3). There are only few cases of TET-associated myocarditis reported in the pre-immunotherapy era (50-56). However, myocarditis (presenting either with symptoms, or isolated troponin elevation) was observed in 5% patients with thymic carcinoma and 43–57% patients with thymoma enrolled in clinical trials and treated with ICIs (33,34,41). The occurrence of myocarditis as a complication of ICI therapy in patients with thymoma has also been documented in case reports (57,58). In contrast, the risk of ICI-induced myocarditis in non-TET patients is less than 1% (47). Thus, TET patients, especially those with thymoma, appear to be at an increased risk of developing myocardial inflammation, in addition to effects on skeletal muscle and the neuromuscular junction.
Other immune-related AEs
The risk of irAEs affecting most other organ systems, such as the lungs, gastrointestinal tract, skin and endocrine organs, does not appear to be substantially higher in TET patients when compared with patients with other cancers (33,34,41). Pure red cell aplasia is a potential exception and it has been reported in patients with thymoma following treatment with a WT-1 peptide-based vaccine and after treatment with other immunomodulatory drugs (7,43,49).
Strategies to reduce the risk of irAEs in TET patients
Since thymic biology places TET patients at an increased risk for irAEs, it is necessary to develop strategies that can identify and treat patients at risk, if immunotherapy is to be developed as a feasible treatment option for these patients. In addition to defective immune tolerance associated with TETs, prior treatments, including chemotherapy, radiation therapy and targeted biologic therapies also have the potential to reshape patients’ immune profiles and increase the risk of developing irAEs (59). These observations provide an impetus towards developing risk mitigation strategies to enhance the safety of immunotherapy in the TET population. The following considerations merit discussion.
First, prior to initiation of immunotherapy, is it possible to identify TET patients with no clinical history of paraneoplastic autoimmunity who might be at risk for developing irAEs? Our group recently reported that pre-existing anti-acetylcholine receptor (anti-AchR) autoantibodies and B cell lymphopenia put thymoma patients at high risk for developing myositis after treatment with avelumab (60). Four out of seven thymoma patients had measurable anti-AchR autoantibodies at baseline, all of whom developed myositis upon treatment. In contrast, patients without anti-AchR autoantibodies did not develop myositis after receiving avelumab. Three of four patients who developed myositis also had pre-treatment anti-striational antibodies, whereas these antibodies were absent in patients who did not develop myositis. In addition, thymoma patients who developed myositis had profound B cell lymphopenia at baseline (B cells comprised 0.19% of peripheral blood mononuclear cells) compared with TET patients who did not develop myositis (12.3%), individuals with non-thymic malignancies (8.3%) and age-matched healthy controls (16.3%). This finding is consistent with a recent report of treatment-induced B cell changes correlating with irAEs in patients with melanoma receiving ICIs (61). These observations merit further investigation and should prompt consideration of avoiding immunotherapy in thymoma patients with anti-AchR autoantibodies and severe B-cell lymphopenia even in the absence of a clinical history of paraneoplastic autoimmune disease.
Second, does secondary prophylaxis with an immunosuppressive drug and re-challenge with immunotherapy have a role in TET patients after successful treatment of an initial non-life-threatening irAE? In a retrospective series of 93 patients treated with an anti-PD-1/PD-L1 ICI and experiencing an initial grade 2 or higher irAE, 40 (43%) patients were rechallenged with the same drug. The same irAE or a different irAE was observed in 22 (55%) patients (62). Recurrence of an irAE was associated with a short interval between initiation of ICI therapy and development of the initial irAE and the second event was not as severe as the initial event. Successful resumption of ICI therapy has also been described in patients receiving combination anti-CTLA-4 and anti-PD-1 therapy and in two patients who developed immune-related myositis (63,64). These results show that ICI rechallenge appears potentially feasible, although the decision to reintroduce an ICI should be taken in the context of several factors including the benefit derived from initial therapy, and the type and severity of the initial irAE. ICI rechallenge is generally not recommended if a patient develops severe myositis, myocarditis, neurological or neuromuscular complications after initial treatment. The conditions under which an ICI can be reintroduced after an irAE needs further evaluation in a prospective clinical trial, especially in TET patients who are predisposed to severe and atypical irAEs. Future clinical trials also need to incorporate strategies for monitoring immune activation and the clinical activity of an irAE in TET patients who are considered for ICI rechallenge, with a low threshold to discontinue treatment upon recurrence of an irAE. The question of secondary prophylaxis with an immunosuppressive drug prior to ICI rechallenge has not been addressed thus far and needs further investigation. Use of immunosuppressive drugs for secondary prophylaxis against an irAE is especially complicated since these drugs have the potential to blunt the immune response triggered by the ICI.
Third, since a fairly significant proportion of patients with thymoma (30–40%) have autoimmune disorders at diagnosis, can TET patients with pre-existing paraneoplastic autoimmunity be safely treated with immunotherapy without being placed at risk for a life-threatening flare of the preexisting autoimmune condition upon initiation of immunotherapy? This question also needs to be addressed in future studies since most TET patients with coexisting autoimmune diseases are excluded from ongoing clinical trials. Johnson and colleagues have demonstrated the feasibility of using an ICI to treat patients with preexisting autoimmune disorders. In a study of ipilimumab in 30 patients with advanced melanoma and preexisting autoimmune disorders (including rheumatoid arthritis, inflammatory bowel disease, sarcoidosis, transverse myelitis, psoriasis, and Grave’s disease), treatment exacerbated preexisting autoimmune disorders in eight patients (27%) and new irAEs arose in 10 (33%) patients. However, 15 (50%) patients had neither a flare of preexisting autoimmune disease nor did they develop new irAEs (65). Although the risk of autoimmune exacerbation might be higher in TET patients due to underlying biological differences, these results provide a basis for including TET patients with certain preexisting autoimmune conditions in future immunotherapy trials without putting them at undue risk for development of treatment-related complications.
Future avenues for immunotherapy in TETs
Besides published trials of ICIs in TETs, there are five ongoing clinical trials that are evaluating ICIs alone (avelumab, nivolumab or pembrolizumab) or in combination with other drugs (pembrolizumab with epacadostat or sunitinib) in TET patients (Table 3). Results of these trials are keenly awaited and will provide further information about the risks and benefits of using ICIs, either alone or as part of a combination strategy in patients with TETs.

Full table
In addition to blocking the PD-1/PD-L1 axis, there are multiple co-inhibitory immune checkpoints (LAG3, B7-H3, B7-H4 and TIM-3) and co-stimulatory molecules (CD137, GITR, ICOS) regulating T cell-mediated anti-tumor response (15). A recent study has reported TIM-3 expression in TETs (66), which provides a new target for immune checkpoint inhibition. These results also highlight the need for comprehensive immune profiling of TETs to identify new therapeutic targets.
Conclusions
ICIs have shown clinical activity in relapsed and refractory TETs but are associated with a high risk of precipitating irAEs. Therefore, if ICIs are under consideration for TET patients, it is preferable that treatment be administered in the context of a clinical trial. TET patients with preexisting autoimmune disorders are at very high risk of developing irAEs and should not be treated with immunotherapy until adequate risk mitigation strategies have been developed. Biomarkers for identification of TET patients at risk for irAEs are under development.
Treating TETs with immunotherapy involves a balancing act between inducing a clinical response and precipitating potentially severe irAEs. To make immunotherapy a safe and feasible option for patients with TETs, the aforementioned challenges will have to be overcome before widespread adoption of these treatments.
Acknowledgments
Funding: This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors Mirella Marino and Brett W. Carter for the series “Dedicated to the 9th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2018)” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2019.08.02). The series “Dedicated to the 9th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2018)” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol 2010;5:S260-5. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke A, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed. Lyon, France: International Agency for Research on Cancer, 2015:412.
- Marx A, Willcox N, Leite MI, et al. Thymoma and paraneoplastic myasthenia gravis. Autoimmunity 2010;43:413-27. [Crossref] [PubMed]
- Merveilleux du Vignaux C, Dansin E, Mhanna L, et al. Systemic Therapy in Advanced Thymic Epithelial Tumors: Insights from the RYTHMIC Prospective Cohort. J Thorac Oncol 2018;13:1762-70. [Crossref] [PubMed]
- Rajan A, Giaccone G. Treatment of advanced thymoma and thymic carcinoma. Curr Treat Options Oncol 2008;9:277-87. [Crossref] [PubMed]
- Kelly RJ, Petrini I, Rajan A, et al. Thymic malignancies: from clinical management to targeted therapies. J Clin Oncol 2011;29:4820-7. [Crossref] [PubMed]
- Thomas A, Rajan A, Berman A, et al. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: an open-label phase 2 trial. Lancet Oncol 2015;16:177-86. [Crossref] [PubMed]
- Zucali PA, De Pas T, Palmieri G, et al. Phase II Study of Everolimus in Patients With Thymoma and Thymic Carcinoma Previously Treated With Cisplatin-Based Chemotherapy. J Clin Oncol 2018;36:342-9. [Crossref] [PubMed]
- Chen Y, Gharwan H, Thomas A. Novel biologic therapies for thymic epithelial tumors. Front Oncol 2014;4:103. [Crossref] [PubMed]
- Radovich M, Pickering CR, Felau I, et al. The Integrated Genomic Landscape of Thymic Epithelial Tumors. Cancer Cell 2018;33:244-58.e10. [Crossref] [PubMed]
- Wang Y, Thomas A, Lau C, et al. Mutations of epigenetic regulatory genes are common in thymic carcinomas. Sci Rep 2014;4:7336. [Crossref] [PubMed]
- Forde PM, Reiss KA, Zeidan AM, et al. What lies within: novel strategies in immunotherapy for non-small cell lung cancer. Oncologist 2013;18:1203-13. [Crossref] [PubMed]
- Mandal R, Chan TA. Personalized Oncology Meets Immunology: The Path toward Precision Immunotherapy. Cancer Discov 2016;6:703-13. [Crossref] [PubMed]
- Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol 2018;62:29-39. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350-5. [Crossref] [PubMed]
- Song Y, Li Z, Xue W, et al. Predictive biomarkers for PD-1 and PD-L1 immune checkpoint blockade therapy. Immunotherapy 2019;11:515-29. [Crossref] [PubMed]
- Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. [Crossref] [PubMed]
- Lin H, Wei S, Hurt EM, et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J Clin Invest 2018;128:805-15. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Gandara DR, Paul SM, Kowanetz M, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med 2018;24:1441-8. [Crossref] [PubMed]
- Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019;51:202-6. [Crossref] [PubMed]
- Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960-4. [Crossref] [PubMed]
- Blank CU, Haanen JB, Ribas A, et al. Cancer Immunology. The “cancer immunogram”. Science 2016;352:658-60. [Crossref] [PubMed]
- June CH, Warshauer JT, Bluestone JA. Is autoimmunity the Achilles' heel of cancer immunotherapy? Nat Med 2017;23:540-7. [Crossref] [PubMed]
- Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 2016;54:139-48. [Crossref] [PubMed]
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714-68. [Crossref] [PubMed]
- Patil PD, Burotto M, Velcheti V. Biomarkers for immune-related toxicities of checkpoint inhibitors: current progress and the road ahead. Expert Rev Mol Diagn 2018;18:297-305. [Crossref] [PubMed]
- Weissferdt A, Fujimoto J, Kalhor N, et al. Expression of PD-1 and PD-L1 in thymic epithelial neoplasms. Mod Pathol 2017;30:826-33. [Crossref] [PubMed]
- Katsuya Y, Fujita Y, Horinouchi H, et al. Immunohistochemical status of PD-L1 in thymoma and thymic carcinoma. Lung Cancer 2015;88:154-9. [Crossref] [PubMed]
- Padda SK, Riess JW, Schwartz EJ, et al. Diffuse high intensity PD-L1 staining in thymic epithelial tumors. J Thorac Oncol 2015;10:500-8. [Crossref] [PubMed]
- Sekine I AY, Suzuki H. Expression patterns and prognostic value of programmed death ligand-1 and programmed death 1 in thymoma and thymic carcinoma. Mediastinum 2018;2:54. [Crossref]
- Cho J, Kim HS, Ku BM, et al. Pembrolizumab for Patients With Refractory or Relapsed Thymic Epithelial Tumor: An Open-Label Phase II Trial. J Clin Oncol 2019;37:2162-70. [Crossref] [PubMed]
- Giaccone G, Kim C, Thompson J, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol 2018;19:347-55. [Crossref] [PubMed]
- Heery CR, O'Sullivan-Coyne G, Madan RA, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol 2017;18:587-98. [Crossref] [PubMed]
- Cheng M, Anderson MS. Thymic tolerance as a key brake on autoimmunity. Nat Immunol 2018;19:659-64. [Crossref] [PubMed]
- Anderson MS, Su MA. Aire and T cell development. Curr Opin Immunol 2011;23:198-206. [Crossref] [PubMed]
- Maverakis E, Goodarzi H, Wehrli LN, et al. The etiology of paraneoplastic autoimmunity. Clin Rev Allergy Immunol 2012;42:135-44. [Crossref] [PubMed]
- Weksler B, Lu B. Alterations of the immune system in thymic malignancies. J Thorac Oncol 2014;9:S137-42. [Crossref] [PubMed]
- Kim TK, Herbst RS, Chen L. Defining and Understanding Adaptive Resistance in Cancer Immunotherapy. Trends Immunol 2018;39:624-31. [Crossref] [PubMed]
- Rajan A, Heery CR, Perry S, et al. Safety and clinical activity of anti-programmed death-ligand 1 (PD-L1) antibody (ab) avelumab (MSB0010718C) in advanced thymic epithelial tumors (TETs). J Clin Oncol 2016;34:e20106 [Crossref]
- Chen F, Zou Z, Du J, et al. Neoantigen identification strategies enable personalized immunotherapy in refractory solid tumors. J Clin Invest 2019;129:2056-70. [Crossref] [PubMed]
- Oji Y, Inoue M, Takeda Y, et al. WT1 peptide-based immunotherapy for advanced thymic epithelial malignancies. Int J Cancer 2018;142:2375-82. [Crossref] [PubMed]
- Takahashi N, Zhao C, Rajan A. WT1 as an immunotherapy target for thymic epithelial tumors: a novel method to activate anti-tumor immunity. Mediastinum 2019;3:11. [Crossref] [PubMed]
- Lesueur P, Chevalier F, Stefan D, et al. Review of the mechanisms involved in the abscopal effect and future directions with a focus on thymic carcinoma. Tumori 2017;103:217-22. [Crossref] [PubMed]
- Bernard C, Frih H, Pasquet F, et al. Thymoma associated with autoimmune diseases: 85 cases and literature review. Autoimmun Rev 2016;15:82-92. [Crossref] [PubMed]
- Zimmer L, Goldinger SM, Hofmann L, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer 2016;60:210-25. [Crossref] [PubMed]
- Giaccone G, Rajan A, Berman A, et al. Phase II study of belinostat in patients with recurrent or refractory advanced thymic epithelial tumors. J Clin Oncol 2011;29:2052-9. [Crossref] [PubMed]
- Rajan A, Carter CA, Berman A, et al. Cixutumumab for patients with recurrent or refractory advanced thymic epithelial tumours: a multicentre, open-label, phase 2 trial. Lancet Oncol 2014;15:191-200. [Crossref] [PubMed]
- Saito N, Shimizu K, Kawaishi M, et al. A survival case of invasive thymoma accompanied by acute fulminant myocarditis. Respirol Case Rep 2013;1:36-8. [Crossref] [PubMed]
- Koul D, Kanwar M, Jefic D, et al. Fulminant giant cell myocarditis and cardiogenic shock: an unusual presentation of malignant thymoma. Cardiol Res Pract 2010;2010:185896 [Crossref] [PubMed]
- Venna N, Gonzalez RG, Zukerberg LR. Case records of the Massachusetts General Hospital. Case 39-2011. A woman in her 90s with unilateral ptosis. N Engl J Med 2011;365:2413-22. [Crossref] [PubMed]
- Shah A, Pace A, Hilton D, et al. Giant cell myositis responsive to combined corticosteroids and immunoglobulin. Pract Neurol 2015;15:456-9. [Crossref] [PubMed]
- Kon T, Mori F, Tanji K, et al. Giant cell polymyositis and myocarditis associated with myasthenia gravis and thymoma. Neuropathology 2013;33:281-7. [Crossref] [PubMed]
- Priemer DS, Davidson DD, Loehrer PJ, et al. Giant Cell Polymyositis and Myocarditis in a Patient With Thymoma and Myasthenia Gravis: A Postviral Autoimmune Process? J Neuropathol Exp Neurol 2018;77:661-4. [Crossref] [PubMed]
- Tanahashi N, Sato H, Nogawa S, et al. A case report of giant cell myocarditis and myositis observed during the clinical course of invasive thymoma associated with myasthenia gravis. Keio J Med 2004;53:30-42. [PubMed]
- So H, Ikeguchi R, Kobayashi M, et al. PD-1 inhibitor-associated severe myasthenia gravis with necrotizing myopathy and myocarditis. J Neurol Sci 2019;399:97-100. [Crossref] [PubMed]
- Chen Q, Huang DS, Zhang LW, et al. Fatal myocarditis and rhabdomyolysis induced by nivolumab during the treatment of type B3 thymoma. Clin Toxicol (Phila) 2018;56:667-71. [Crossref] [PubMed]
- Hodge JW, Ardiani A, Farsaci B, et al. The tipping point for combination therapy: cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Semin Oncol 2012;39:323-39. [Crossref] [PubMed]
- Mammen AL, Rajan A, Pak K, et al. Pre-existing antiacetylcholine receptor autoantibodies and B cell lymphopaenia are associated with the development of myositis in patients with thymoma treated with avelumab, an immune checkpoint inhibitor targeting programmed death-ligand 1. Ann Rheum Dis 2019;78:150-2. [Crossref] [PubMed]
- Das R, Bar N, Ferreira M, et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J Clin Invest 2018;128:715-20. [Crossref] [PubMed]
- Simonaggio A, Michot JM, Voisin AL, et al. Evaluation of Readministration of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients With Cancer. JAMA Oncol 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Pollack MH, Betof A, Dearden H, et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol 2018;29:250-5. [Crossref] [PubMed]
- Delyon J, Brunet-Possenti F, Leonard-Louis S, et al. Immune checkpoint inhibitor rechallenge in patients with immune-related myositis. Ann Rheum Dis 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Johnson DB, Sullivan RJ, Ott PA, et al. Ipilimumab Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA Oncol 2016;2:234-40. [Crossref] [PubMed]
- Arbour KC, Naidoo J, Steele KE, et al. Expression of PD-L1 and other immunotherapeutic targets in thymic epithelial tumors. PLoS One 2017;12:e0182665 [Crossref] [PubMed]
Cite this article as: Zhao C, Rajan A. Immune checkpoint inhibitors for treatment of thymic epithelial tumors: how to maximize benefit and optimize risk? Mediastinum 2019;3:35.


