
Primary mediastinal chondrosarcomas: do they really exist?
Introduction
Primary chondrosarcomas arising in the mediastinal compartment are exceedingly rare. However, such reports have been presented in the literature predominantly as either small series of cases or single case reports (1-19). The issue of primary cartilaginous tumors in the mediastinum should be viewed in the similar context as extra-skeletal chondrosarcomas or osteosarcomas in the soft tissues (20-25). Therefore, it should not be surprising that such tumors, the same as those occurring in the soft tissues, may also originate from the mediastinal compartment. The criteria for such designation in the mediastinum, just as with other anatomic locations, should be that the tumor does not have any connection to any bony structures within the thoracic cavity. In addition, in the mediastinal compartment the criteria can be extended somewhat to include that not only the tumor is not connected to any bony structure but also that the tumor does not arise in the context of any other tumoral condition that may be hosting a malignant cartilaginous component. In this setting of strict criteria and having the benefit of state of the art diagnostic imaging and molecular techniques, the number of cases that may be accepted as truly originating from the mediastinal compartment or truly representing chondrosarcomas, whether in anterior or posterior compartment, is drastically reduced.
We are aware that what has been regarded as mediastinal chondrosarcomas, namely “mesenchymal” or “myxoid” chondrosarcomas are now considered to belong to separate categories and are believed to represent sarcomas of possible uncertain histogenesis or differentiation, as in both of those entities specific molecular findings have been identified. Nevertheless, those entities whether coded as sarcomas of uncertain histogenesis or differentiation, or “mesenchymal” and/or myxoid” chondrosarcomas do rarely occur in the mediastinal compartment and as such will be discussed within this context. Whatever the specific name for those entities goes well beyond the scope of this review.
Diagnostic criteria and histopathological features
Clinical and radiological features
Mediastinal sarcomas in general are rare. Because the mediastinal compartment technically speaking contains the heart, great vessels, portion of the esophagus, the thymus, and adipose tissue, one would expect that any mesenchymal tumor that may present as a mediastinal mass, likely would arise from those structures. Therefore, the wall of the esophagus could be the source of a smooth muscle neoplasm; the wall of a vessel could give rise to a vascular tumor. However, more importantly, since there is abundant adipose tissue in the mediastinum, one could argue that lipomatous tumors not only should be the only ones to be considered primary “mediastinal” tumors but also the ones that should be the leading type of mesenchymal neoplasms in the mediastinal compartment. In reality, many examples of different types of mediastinal sarcomas have been present in the literature throughout the years.
The clinical manifestations of patients with any type of mediastinal mass is that of compression of adjacent structures and consequently these individuals may present with symptoms of dyspnea, cough, chest pain, back pain, or other non-specific symptoms. On the other hand, a number of patients will be asymptomatic and their mediastinal tumor may be discovered during a routine radiographic examination. Contrary to some epithelial neoplasms such as thymomas, any association with myasthenia gravis or any other autoimmune disorders may represent merely a random association. It is important to highlight that chondrosarcomas and osteosarcomas have been described following therapy for other conditions (18,26).
In terms of the diagnosis of mediastinal chondrosarcomas, often the tumors may present as a mediastinal mass; however, the tumor may have an associated periosteal connection, which will make the tumor as of bone origin, despite the presentation of “mediastinal tumor.” In essence those tumors should not be considered as “mediastinal” in origin and raises the importance of a careful radiological assessment.
Radiological features
Internal coarse calcifications in a “ring and arc” pattern on CT images are typical for a chondroid matrix (Figure 1A,B,C,D). The non-calcified portion of the mass shows low attenuation compared with muscle, suggesting the presence of hyaline cartilage (high water content), tumor necrosis, or cystic content (3). The masses are of low to intermediate signal intensity on T1-weighted MR images with foci of mild hyperintensity that represent areas of mineralization. On T2-weighted MR images, the masses are of heterogeneous mixed signal intensity, typically with scattered areas of high signal intensity representing areas of high water content (3). The fibrovascular septae of chondrosarcomas is postulated to account for the lobular morphology allowing for growth of mostly avascular cartilaginous tumor (3). With administration of intravenous contrast, the tumor shows heterogeneous enhancement.
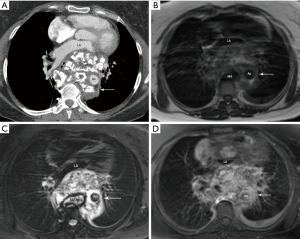
Diagnostic criteria
Based on those restrictions, we consider that the diagnosis of mediastinal chondrosarcomas should adhere to the following guidelines:
- Unequivocal radiographic evidence that the tumor does not have a connection to any bony structure.
- The patient should not have any clinical background of radiation therapy for a mediastinal tumor, of which only biopsy material was evaluated, i.e., mediastinal germ cells tumors treated prior to complete surgical debulking.
- The diagnosis requires complete histopathological assessment of the surgically resected neoplasm.
- Cases in which there is only biopsy material or fine needle aspirated material, although important in the initial diagnosis, the diagnosis should be regarded only as presumptive in such cases, and the final interpretation is rendered only after complete surgical evaluation of the surgically resected neoplasm.
- Extensive sampling of the resected tumor mass is required in order to properly exclude the possibility of another component, whether epithelial or mesenchymal.
- The diagnostic criteria for mediastinal chondrosarcoma should be the same as the criteria employed in conventional tumors of the bone.
We consider that adhering to these particular guidelines will provide the most important parameters that can be used in the final interpretation of mediastinal chondrosarcomas.
Histopathological features
As stated before, diagnostic criteria for the diagnosis of mediastinal chodrosarcomas should be the same as that use for similar tumors in the bone. Just as tumors in the bone, the tumors that have been described in the mediastinum show similar characteristics and display the entire gamut of histopathological growth patterns. Descriptions of chondrosarcomas have included: conventional chondrosarcomas as well as myxoid and mesenchymal chondrosarcomas.
Chondrosarcomas
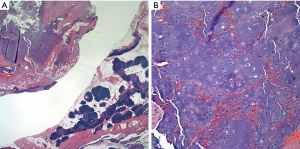
Historically, the subtypes of chondrosarcomas described in the mediastinum show the same spectrum as those that have been described in bone. Grossly, when these tumors occur in the anterior mediastinal compartment, they appear to be larger than those in the posterior mediastinum (27,28). In addition, it appears that those in the anterior mediastinum are rather well circumscribed but not encapsulated. Those in the posterior mediastinum may show less circumscription and may also have ill-defined borders. The histological features of these tumors span from the low-grade to the high-grade chondrosarcoma in which the tumor may show lobulation and mild nuclear atypia, to the higher-grade tumors, where nuclear atypia is marked and it is possible to identify mitotic activity (Figure 2A,B).
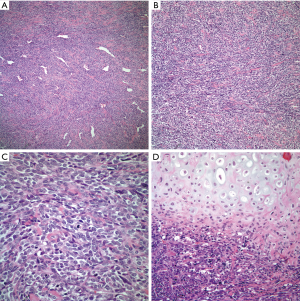
On the other hand, tumors have also been coded under the designation of mesenchymal chondrosarcoma. In these cases, the tumor may show only focal areas in which cartilage is present. At low power view, the tumor characteristically shows a subtle hemangiopericytic growth pattern with the presence of large dilated vessels. In other areas, the tumor may show sheets of neoplastic cells without any particular growth pattern. At higher magnification, the tumor is composed of rather small cells with scant cytoplasm, small round nuclei and inconspicuous nucleoli (Figure 3A,B,C,D). Nuclear atypia is marked and mitotic activity is easily identified. Also, one other variant of chondrosarcoma that has been described in the mediastinum is the so called extra skeletal myxoid chondrosarcoma in which there is not well-formed cartilage and the tumor cells appear somewhat epithelioid with scant cytoplasm, small oval nuclei and inconspicuous nucleoli embedded in a myxoid matrix. Although these tumors may not show marked cellular atypia, the presence of mitotic activity is easily identified.
As stated earlier, these two latter tumors appear to have specific molecular characteristics, which have suggested that they may not represent chondrosarcomas but rather sarcomas of uncertain differentiation.
Immunohistochemical and molecular features
The use of immunohistochemical studies in the diagnosis of chondrosarcoma of the mediastinum is limited and oftentimes, the immunohistochemical stains are employed essentially to rule out the possibility of another neoplasm. Positive immunohistochemical stains in chondrosarcoma will include S-100 protein, SOX4, and SOX9. Shon et al. (29) evaluated 111 bone and soft tissue tumors with chondroid differentiation using an anti-ERG monoclonal antibody directed against the N terminus. The authors identified that two of four cases of “mesenchymal” chondrosarcoma the hyaline cartilage showed positivity, while variable positivity was observed in four out of nine cases of extra-skeletal “myxoid” chondrosarcoma. Recently, Jeong and Kim (30) presented a review on the topic of biomarkers of chondrosarcomas and elaborated on different biomarkers that have been identified with possible prognostic and treatment implications. Some of these biomarkers include: leptin and adiponectin, which are cytokines that may be expressed in chondrosarcomas. Both of those cytokines may be used in staging of chondrosarcomas. Periostin is a stromal-related protein that appears to be present in low-grade chondrosarcomas. Vascular endothelial growth factor A and C (VEGF-A and C) also appear to have a role in the grade of this tumor. Sirtuin-1 (SIRT) expression in chondrosarcoma appears to correlate with poor prognosis. On the other hand, Survivin, which is an inhibitor of apoptosis may represent a therapeutic target and a predictive biomarker.
Regarding molecular features, mesenchymal chondrosarcomas will show HEY1-NCOA2 gene fusion, while myxoid chondrosarcomas will show NR4A3 gene rearrangement. Therefore, it has been suggested that these two tumoral conditions that in the past have been coded as “mesenchymal” and “myxoid” chondrosarcomas, in the era of molecular characterization of tumors, may not correspond to the family of “chondrosarcomas.”
Discussion
The presence of mediastinal tumors in which there is a component of either benign or malignant cartilage has been well described in the literature in a diversity of mediastinal tumors (25,26,31). In addition, it is well known that some tumors such as mediastinal germ cell tumors, especially teratomas may also show the presence of malignant mesenchymal component, which may be in the form of malignant cartilage or bone (32). However, it is important to state that the current treatment of mediastinal germ cell tumors is that of chemotherapy followed by debulking of the mediastinal mass. Such information becomes important as the mode of therapy may obliterate any epithelial component but not so of the chondroid component of the tumor, which in turn may be misinterpreted as post-treatment chondrosarcoma. We consider that such tumors likely represent somatic transformation of the original germ cell tumors and not a de novo mediastinal sarcoma. One other way in which chondrosarcoma may be misconstrued as originating from the mediastinal compartment is when the tumor despite its presentation as “mediastinal mass,” is indeed originating from another thoracic structure such the rib, cricoid cartilage, larynx, or the spine for those tumors in the posterior mediastinum (33-35). Therefore, following strict clinical criteria, the actual number of cases that may meet such strict criteria and can be truly regarded as primary to the mediastinum, become the minority of cases. In today’s practice, the use of state of the art diagnostic imaging should leave no doubt as to where the tumor is located and its association with adjacent structures. If in addition to the clinical criteria, we add the extensive sampling that these tumors should undergo when in the mediastinum to properly exclude the possibility of another either epithelial or mesenchymal component, then we will likely reduce the number of acceptable cases.
At this juncture, it is important to highlight a few critical issues regarding the current knowledge of chondrosarcomas. Retrospective molecular characterization of tumors that over the years had been designated as mesenchymal or myxoid chondrosarcomas, suggests that these two particular entities may represent separate tumoral conditions. For instance, extra-skeletal myxoid chondrosarcomas, which has the characteristic of NR4A3 gene rearrangement, is now considered a rare sarcoma of uncertain differentiation (36). In addition, the HEY1-NCOA2 fusion that is present in what has been coded under mesenchymal chondrosarcoma appears to be unique to this particular tumor (37). Furthermore, what is regarded as “conventional” chondrosarcoma appears to harbor in more than 50% of the cases a gene mutation in the metabolic enzymes isocitrate dehydrogenase 1 and 2 (IDH1/IDH2) (38). It is in this background of current knowledge that what might have been described, as “chondrosarcoma” in the mediastinum in past literature, currently may not be viewed in the same light. Therefore, as those entities previously described are addressed in this review, it is in the context of the specific histopathological features that those tumors have. Whether they are sarcomas of uncertain differentiation or sarcomas of specific lineage is beyond the discussion of the occurrence of these tumors in the mediastinal compartment.
From the clinical point of view, mediastinal chondrosarcomas have been described in a wide spectrum of ages—from the young to the adult. In addition, the tumors do not appear to have predilection for specific gender. Although in some cases, such diagnosis has been followed after treatment for another condition, there does not appear to be a specific medical condition that may be associated with either one of those tumors. The symptomatology recorded in some of these patients is essentially non-specific as it may be seen in associated with any mass in the anterior or posterior compartment. In terms of the histological spectrum of chondrosarcomas in the mediastinum, this tumor has been described in either anterior or posterior location. However, it appears that what has been regarded as mesenchymal chondrosarcomas may favor the posterior mediastinum while the more conventional chondrosarcomas are more common in the anterior mediastinum. This anatomic distribution coupled with the fact that chondrosarcomas have been described in a wide range of age groups, forces the consideration of different conditions in the differential diagnosis. For instance, the occurrence of “mesenchymal” chondrosarcoma in children will raise the possibility of different tumors that may occur in this group, namely small round cell tumors, which will include primitive neuroectodermal tumor (PNET), rhabdomyosarcoma, and neuroblastoma/ganglioneuroblastoma. The use of immunohistochemical stains may provide some aid in leaning towards a more specific diagnosis as these tumors will likely stain with some markers that will be helpful in their separation. Also molecular diagnostics may be of help in cases in which the morphology and immunohistochemistry are problematic. In cases of rhabdomyosarcoma, the use of muscle markers such desmin and Myo-D will provide aid in the separating rhabdomyosarcoma from a mesenchymal chondrosarcoma. In cases of PNET or neuroblastoma, there is not one specific immunohistochemical stain that can be stated as pathognomonic for these tumors but rather the use of several stains that may aid in the diagnosis, in which case the use of synaptophysin and CD99 may be of helpful. Also, it is important to highlight that S-100 protein may show positive staining in mesenchymal chondrosarcoma while may only be focally or weakly positive in PNET and neuroblastoma. However, the molecular signature for PNET is different, while the amplification of Myc in neuroblastomas is of aid.
On the contrary, the occurrence of conventional chondrosarcoma in the anterior mediastinum in a young male patient raises the possibility of a germ cell tumor with somatic transformation. This in essence brings a different problem. Although cases of conventional chondrosarcomas in the anterior mediastinum have been described, it is essential that the tumor not only be completely resected but also be extensively sampled in order to avoid miscoding the case. In our experience, this type of chondrosarcoma, even though it may be not so difficult to diagnose from the histopathological point of view, it is the one that offers more problems in interpretation of cases that are not properly sampled.
Although it is reasonable to consider that the clinical outcome of patients with mediastinal chondrosarcomas be similar to their extra-skeletal counterparts, the fact is that there are not enough cases that meet strict criteria to make a meaningful conclusion. Even though, some cases have followed an aggressive behavior with a fatal outcome, in other cases longer survivals have been recorded. Some authors have suggested that patients with chondrosarcomas in the anterior mediastinum may observe a longer survival. Needless to say, metastatic disease has also been recorded in some of the cases described. Once again, with the current knowledge of chondrosarcomas, some of these entities—mesenchymal and myxoid likely follow a different clinical outcome and are treated differently.
Summary
In today’s practice with the use of molecular techniques applied for further characterization of neoplasms, the current knowledge of molecular characterization of chondrosarcomas highlights the important issue of proper classification and raises an important issue: how to designate those tumors, which with no doubt occur in extraskeletal location—mediastinum, which in the past were coded/diagnosed as mesenchymal or myxoid chondrosarcomas? If to that fact, we add the strict radiological and histopathological criteria, then we likely end with a very limited number of cases that could be regarded as true mediastinal chondrosarcomas.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Saul Suster and David Suster) for the series “Mediastinal Sarcomas” published in Mediastinum. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-38). The series “Mediastinal Sarcomas” was commissioned by the editorial office without any funding or sponsorship. CAM serves as an unpaid editorial board member of Mediastinum from Feb 2018 to Jan 2020. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Phillips GWL, Choong M. Case report: chondrosarcoma presenting as an anterior mediastinal mass. Clin Radiol 1991;43:63-4. [Crossref] [PubMed]
- Østergaard ML, Petersen RH, Kalhauge A. A chondrosarcoma in the anterior mediastinum mimicking a thymoma. Acta Radiol Open 2015;4:1-3. [Crossref] [PubMed]
- Nasseri F, Chen GJ, Nachiappan AC. Case 195: chondrosarcoma of the posterior mediastinum. Radiology 2013;268:299-303. [Crossref] [PubMed]
- Pescarmona E, Rendina EA, Venuta F, et al. Myxoid chondrosarcoma of the mediastinum. Appl Pathol 1989;7:318-21. [PubMed]
- Webster BH. Chondrosarcoma of the posterior mediastinum: a case simulating a neurofibroma. Dis Chest 1957;31:104-8. [Crossref] [PubMed]
- Sullivan JJ, Mangiardi JL. Chondrosarcoma in the posterior mediastinum. J Thorac Surg 1958;36:744-8. [Crossref] [PubMed]
- Montresor E, Abrescia F, Bertrand C, et al. Mediastinal chondrosarcoma. Case report. Acta Chir Scand 1990;156:733-6. [PubMed]
- Suster S, Moran CA. Malignant cartilaginous tumors of the mediastinum: clinicopathologic study of six cases presenting as extraskeletal soft tissue masses. Hum Pathol 1997;28:588-94. [Crossref] [PubMed]
- Chetty R. Extraskeletal mesenchymal chondrosarcoma of the mediastinum. Histopathology 1990;17:261-3. [Crossref] [PubMed]
- Ratto GB, Costa R, Alloisio A, et al. Mediastinal chondrosarcoma. Tumori 2004;90:151-3. [Crossref] [PubMed]
- Jeong SS, Choi PJ, Kim DW, et al. Primary extraskeletal mesenchymal chondrosarcoma of the anterior mediastinum. Korean J Pathol 2013;47:492-4. [Crossref] [PubMed]
- Ikeda T, Ishihara T, Yoshimatsu H, et al. Primary osteogenic sarcoma of the mediastinum. Thorax 1974;29:582-8. [Crossref] [PubMed]
- Tarr RW, Kerner T, McCrook B, et al. Primary extraosseous osteogenic sarcoma of the mediastinum: clinical, pathologic, and radiologic correlation. South Med J 1988;81:1317-9. [Crossref] [PubMed]
- Greenwood SM, Meschter SC. Extraskeletal osteogenic sarcoma of the mediastinum. Arch Pathol Lab Med 1989;113:430-3. [PubMed]
- De Nictolis M, Goteri G, Brancorsini D, et al. Extraskeletal osteosarcoma of the mediastinum associated with long-term patient survival. A case report. Anticancer Research 1995;15:2785-9. [PubMed]
- Hishida T, Yoshida J, Nishimura M, et al. Extraskeletal osteosarcoma arising in anterior mediastinum: brief report with a review of the literature. J Thorac Oncol 2009;4:927-9. [Crossref] [PubMed]
- Yu H, Wu Z, Cui Y, et al. Low-grade extraskeletal osteosarcoma of the mediastinum: report of a case and review of literature. Int J Clin Exp Pathol 2015;8:3279-81. [PubMed]
- Catanese J, Dutcher JP, Dorfman HD, et al. Mediastinal osteosarcoma with extension to lungs in a patient treated for Hodgkin’s disease. Cancer 1988;62:2252-7. [Crossref] [PubMed]
- Faz GT, Eltorky M, Karnath B. Concurrent nontuberculous mycobacteria infection and high-grade anterior mediastinal extraskeletal osteosarcoma (ESOS): is there a connection? Am J Case Rep 2016;17:592-6. [Crossref] [PubMed]
- Nakashima Y, Unni KK, Shives TC, et al. Mesenchymal chondrosarcoma of bone and soft tissue: a review of 111 cases. Cancer 1986;57:2444-53. [Crossref] [PubMed]
- Bane BL, Evans HL, Ro JY, et al. Extraskeletal osteosarcoma. A clinicopathologic review of 26 case. Cancer 1990;65:2762-70. [Crossref] [PubMed]
- Torigoe T, Yazawa Y, Takagi T, et al. Extraskeletal osteosarcoma in Japan: multiinstitutional study of 20 patients from the Japanese Musculoskeletal Oncology Group. J Orthop Sci 2007;12:424-9. [Crossref] [PubMed]
- Cho SJ, Horvai A. Chondro-osseous lesions of soft tissue. Surg Pathol Clin 2015;8:419-44. [Crossref] [PubMed]
- Lee JS, Fetsch JF, Wasdhal DA, et al. A review of 40 patients with extraskeletal osteosarcoma. Cancer 1995;76:2253-9. [Crossref] [PubMed]
- Qian J, Zhang XY, Gu P, et al. Primary thoracic extraskeletal osteosarcoma: a case report and literature review. J Thorac Dis 2017;9:E1088-95. [Crossref] [PubMed]
- Ulusakarya A, Terrier P, Regnard JF, et al. Extraskeletal osteosarcoma of the mediastinum after treatment of a mediastinal germ-cell tumor. Am J Clin Oncol 1999;22:609-14. [Crossref] [PubMed]
- Nosotti M, Rosso L, Mendogni P. Sternal reconstruction for unusual chondrosarcoma: innovative technique. J Cardiothorac Surg 2012;7:40. [Crossref] [PubMed]
- Weisel W, Ross WB. Chondrosarcoma of the posterior mediastinum with hourglass involvement of the spinal canal: resection and recovery, report of a case. J Thorac Surg 1950;19:643-8. [Crossref]
- Shon W, Folpe AL, Fritchie KJ. ERG expression in chondrogenic bone and soft tissue tumours. J Clin Pathol 2015;68:125-9. [Crossref] [PubMed]
- Jeong W, Kim HJ. Biomarkers of chondrosarcoma. J Clin Pathol 2018;71:579-83. [Crossref] [PubMed]
- Lim YC. Mediastinal chondrolipoma. Am J Surg Pathol 1980;4:407-9. [Crossref] [PubMed]
- Widdowson DJ, Lewis-Jones HG. A large soft-tissue chondroma arising from the posterior mediastinum. Clin Radiol 1988;39:333-5. [Crossref] [PubMed]
- Shrivastava V, Vundavalli S, Smith D, et al. A chondroma of the anterior mediastinum. Clin Radiol 2006;61:1065-6. [Crossref] [PubMed]
- Moran CA, Suster S. Primary germ cell tumors of the mediastinum: I. Analysis of 322 cases with special emphasis on teratomatous lesions and a proposal for histopathologic classification and clinical staging. Cancer 1997;80:681-90. [Crossref] [PubMed]
- Guillem P, Porte H, Copin MC, et al. A case of giant chondrosarcoma of the cricoid cartilage presenting as a superior mediastinal tumor. Eur J Cardiothorac Surg 1998;14:520-2. [Crossref] [PubMed]
- Brenca M, Stacchiotti S, Fassetta K, et al. NR4A fusion proteins trigger an axon guidance switch that marks the difference between EWSR1 and TAF15 translocated extraskeletal myxoid chondrosamas. J Pathol 2019;249:90-101. [Crossref] [PubMed]
- Wang L, Motoi T, Khanin R, et al. Identification of a novel, recurrent HEY1-NCOA2 fusion in mesenchymal chondrosarcoma based on a genome-wide screen of exon-level expression data. Genes Chromosomes Cancer 2012;51:127-39. [Crossref] [PubMed]
- Kerr DA, Lopez HU, Deshpande V, et al. Molecular distinction of chondrosarcoma from chondroblastic osteosarcoma. Am J Surg Pathol 2013;37:787-95. [Crossref] [PubMed]
Cite this article as: Zaleski MP, Truong M, Moran CA. Primary mediastinal chondrosarcomas: do they really exist? Mediastinum 2020;4:24.


