
Mediastinal tumors of peripheral nerve origin (so-called neurogenic tumors)
Introduction
The mediastinum can be the site of origin of a variety of benign and malignant tumors of peripheral nerve origin that have been usually reported as “mediastinal neurogenic tumors” (MNT) (1-4). Anatomically, the mediastinum can be compartmentalized into anterior, middle, and posterior regions (5-12), which can be useful in prioritizing differential diagnostic entities when evaluating mass lesions with the assistance of radiologic correlation. The vast majority of MNT’s arise in the posterior mediastinum, typically arising from the spinal nerve roots, although they may develop from any intrathoracic nerve (1), such as the vagus nerve, or recurrent laryngeal nerve in the superior mediastinum. The majority of MNT’s occur in adult patients, but pediatric cases are described (3,4,13). Table 1 summarizes the various peripheral nerve origin tumors that have been described in the mediastinum.

Full table
Benign tumors of peripheral nerve origin
Schwannoma
Schwannoma is the most frequent tumor of peripheral nerve origin in the mediastinum (14-24) and the majority occur in the posterior mediastinum (25,26). Schwannomas develop as sporadic tumors in children or adults but are more frequent in adult patients in their 3rd–6th decades of life. There is no gender preference. To our knowledge, there have been no mediastinal schwannomas described in patients with schwannomatosis or neurofibromatosis. Patients can be asymptomatic or present with chest pain or symptoms secondary to the extrinsic compression of airways or the esophagus by a large mediastinal tumor (5,14,27). Rarely schwannoma patients present with Horner’s syndrome, pleural effusion, mediastinal hemorrhage or inappropriate secretion of antidiuretic hormone syndrome (IADH syndrome) (16,28-31). Vijendra et al. described an unusual schwannoma of the vagal nerve extending from the mediastinum into the base of the skull (32).
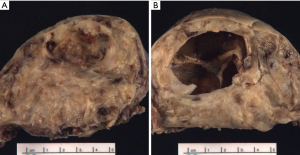
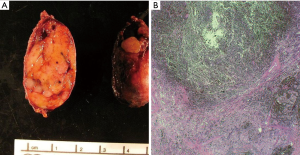
Schwannomas present grossly as well-encapsulated nerve sheath tumors. On cross section they exhibit a yellow-white appearance that is often associated with hemorrhage and/or cystic change (Figure 1A,B). They vary in size from a few centimeters in diameter to “giant” lesions measuring 20 cm in largest dimension (32-34).
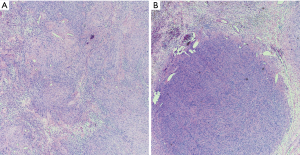
Microscopically, schwannomas exhibit a distinct fibrous capsule that arises from epineurium and usually exhibit focal residual nerve fibers. They are composed of two alternating components: cellular spindle cell areas (Antoni A areas) and loose myxoid areas (Antoni B areas) (Figure 2). The Antoni A areas of schwannomas are composed of spindle cells showing elongated nuclei with inconspicuous nucleoli, amphophilic cytoplasm and indistinct cellular borders. The spindle cells are arranged in short fascicles or whorls and characteristically exhibit nuclear palisading and variable number of Verocay bodies. The latter are composed of two compact rows of well-aligned spindle-shaped nuclei separated by fibrillary cell processes. The Antoni B areas of schwannomas are composed of loose myxoid tissue that often exhibits xanthomatous change, areas of hyalinization and/or cystic change. The hyalinized stroma often exhibits characteristic ectatic, irregularly shaped vessels that can become focally thrombosed. Mitoses are infrequent in schwannomas. Rarely, schwannomas exhibit the presence of focal, benign glands. The spindle cells of schwannomas exhibit, particularly in Antoni A areas, diffuse and often strong cytoplasmic immunoreactivity for S100 protein and nuclear immunoreactivity for SOX-10, a marker of neural crest differentiation. Schwannomas can also exhibit cytoplasmic immunoreactivity for CD57 and glial fibrillary acidic protein (GFAP).
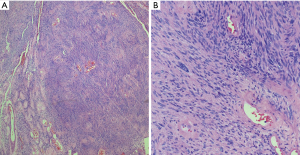
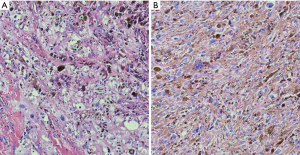
Schwannomas can exhibit extensive areas of degeneration with marked nuclear atypia, hemosiderosis, cystic change, hyalinization, hemorrhage and/or calcification, particularly in patients with longstanding tumors (so-called ancient change) (Figure 3) (17,19,35,36). Schwannomas with ancient change exhibit atypical cells with large hyperchromatic and frequently multilobed nuclei with occasional eosinophilic inclusions (Figure 4). Schwannomas showing ancient change can pose a diagnostic challenge in distinguishing them from other tumors, most importantly those which show more aggressive or malignant biologic behavior, such as malignant peripheral nerve sheath tumors (MPNST) or pleomorphic hyalinizing angiectatic tumor. However, they lack mitotic activity and the presence of highly cellular areas and they maintain the thick-walled hyalinized vessels and foam cells. In addition, this variant of schwannoma maintains its strong diffuse S100 positivity by immunohistochemistry, which helps distinguish it from the above histologic mimics.
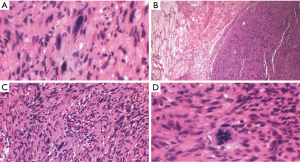
A rare variant of schwannoma can also exhibit areas of pigmentation with melanocytic cells, and rare cases of melanotic schwannoma have been described in the mediastinum (37) (Figures 5,6). To our knowledge other schwannoma variants such as cellular schwannoma and epithelioid schwannoma have not been described as mediastinal lesions.
Schwannomas are benign tumors that are cured by surgical resection (14,38). Depending on the tumor location, the tumors can be resected with thoracotomy, sternotomy, supraclavicular excision, posterior approach with laminectomy, video-assisted thoracoscopic surgery or other techniques (14). Preoperative embolization of giant thymomas has also been used to facilitate tumor resection (21). To our knowledge, there have been no reports of mediastinal schwannomas undergoing malignant transformation after successful resection.
Ganglioneuroma
Ganglioneuromas are neoplasms arising from the dorsal root ganglion of the spinal cord and can grow almost anywhere along the paravertebral sympathetic ganglia and in the adrenal medulla. They can arise de novo and result from the maturation of ganglioneuroblastoma or neuroblastoma. They are rare benign fully differentiated tumors composed of spindled Schwannian or fibroblastic cells, ganglion cells, and nerve fibers without immature elements, atypia, significant mitoses, or intermediate cells. Most cases have been described in the posterior mediastinum of children (39,40). Lee et al. described a patient with a posterior mediastinal ganglioneuroma in a patient with neurofibromatosis (41). The histopathologic features are dependent upon the admixture of elements described above and the immunophenotype is similar to that seen in schwannomas, admixed with scattered ganglion cells. Ganglioneuromas are cured by surgical resection, and to our knowledge there have been no reports of mediastinal lesions progressing to a malignancy (13,39).
Neurofibroma and neurofibromatosis 1 (NF1)
Neurofibromas are benign tumors of peripheral nerve origin that can present as localized, diffuse or plexiform lesions (26). Localized neurofibromas usually develop as sporadic tumors in patients that do not have NF1. Diffuse neurofibromas are an uncommon but distinctive variant of neurofibroma, usually occurring in young adults. It is unclear how often diffuse neurofibromas are associated with neurofibromatosis, though many experts suggest that about 10% of patients with diffuse neurofibromas have NF1, and some reviews suggest that up to 60% of neurofibroma patients exhibit diffuse neurofibromas. Diffuse neurofibromas appear as plaque-like lesions in the head and neck area of children (26). To our knowledge, they have not been reported in the mediastinum. In contrast, plexiform neurofibromas are essentially pathognomonic of NF1. Most mediastinal neurofibromas have been plexiform neurofibromas arising in NF1 patients (42-45). Neurofibromas usually occur in patients in their 3rd and 5th decade of life, without gender predominance. Neurofibromas associated with NF1 are usually diagnosed in children or young adults.
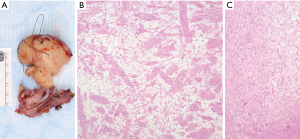
Localized, solitary neurofibromas usually appear a superficial, subcutaneous tumors and only rare examples have been reported in the mediastinum of patients without NF1 (44,46). Al-Hajjaj et al. reported a rare patient with Ehler-Danlos syndrome that presented with a mediastinal neurofibroma and bronchiectasis (47). Localized neurofibromas appear grossly as localized, fusiform lesions that expand the nerve of origin (Figure 7 A,B,C). Microscopically, they are composed of interlacing bundles of spindle cells showing hyperchromatic, wavy nuclei and amphophilic cytoplasm, admixed with ropey, wire like strands of collagen, dense collagen bundles, and variable amounts of intercellular myxoid stroma. Variable amounts of mast cells can also be seen. The spindle cells exhibit a distinctive Schwannian morphologic and immunohistochemical phenotype, with S100 protein and SOX-10 immunoreactivity. However, the proportion of cells and intensity of such immunoreactivity is decreased compared to what is characteristic of schwannomas. Neurofibromas are benign lesions that can be cured with surgical resection, anatomic constraints permitting; however, diffuse and plexiform neurofibromas may inextricably surround vital structures, limiting complete excision.
Most mediastinal neurofibromas are plexiform neurofibromas and several patients with mediastinal either single or bilateral lesions arising from the vagus nerve have been described (42-45). These tumors are pathognomonic of NF1 and develop in early childhood as widely distributed growths within the subcutaneous tissue of extremities and/or deep sites (26). The mediastinal tumors appear as large lesions that present as multiple areas of ill-circumscribed enlargement that deform the nerve of origin into a pattern described as a “bag of worms”. The diagnosis of plexiform neurofibroma is usually made on clinical grounds and correlation with the history of NF1, clinical presentation and radiologic features is essential for making the diagnosis. Microscopically, the lesions show expanded and tortuous nerve branches showing separation of small nerve fibers by endoneurial matrix material with a myxoid appearance (Figure 8). Because plexiform neurofibromas can become cellular and show atypical changes and undergo malignant transformation to MPNST (estimated to be 2% to 29% of cases (48). The histopathologic distinction between atypia and truly malignant transformation in a neurofibroma is notoriously difficult, due to the continuum of histopathologic changes between benign and malignant tumors. Miettinen et al. has proposed to classify these lesions as either atypical neurofibromatous neoplasm of uncertain biologic potential (ANNUBP) or low grade MPNST (49). ANNUBP are diagnosed in the presence of at least two of the following features: high cellularity, cytologic atypia, loss of neurofibroma architecture and/or 2–3 mitoses/10 high power field (HPF). Low grade MPNST's exhibit similar features to ANNUP but with a higher mitotic rate of 3–9 mitoses/10 HPF. Low grade MPNST’s usually exhibit a number of genetic abnormalities, including p53, p16INKARF, p14ARF, and p27kip1 alterations and EGFR amplification (26). Compared to localized and diffuse neurofibromas, the plexiform subtypes have the highest risk of malignant transformation to malignant peripheral nerve sheath tumor (26). Mediastinal plexiform neurofibromas are difficult to treat surgically and patients that develop high grade MPNST have a poor prognosis (42,48).
Granular cell tumor
Several cases of benign and malignant granular cell tumors have been described in the mediastinum (50-57). The tumors develop as well-circumscribed lesions exhibiting a pale-yellow cut surface and are composed of round, polygonal or spindle cells characterized by the presence of abundant eosinophilic, granular cytoplasm. The cells exhibit multiple diastase resistant periodic acid-Schiff (D-PAS) granules and exhibit immunoreactivity for S100, consistent with neural origin and CD68 (Kp1) indicative of lysosomal granules. Granular cell tumors also exhibit nuclear immunoreactivity for TFE3 and Inhibin-alpha. The vast majority of granular cell tumors are benign, but histologically, they can exhibit a range of mild to moderate nuclear atypia. Histologic criteria for malignancy in granular cell tumors includes the presence of spindle cells with vesicular nuclei and prominent nucleoli, necrosis, >2 mitoses/10 HPF, high nuclear/cytoplasmic ratio and cellular pleomorphism; however, in the absence of metastasis clinically malignant behavior remains uncertain because histology is not well correlated to biologic behavior in this set of tumors. Rarely histologically typical granular cell tumors may behave in a malignant fashion. Benign granular cell tumors are cured by surgical resection. Malignant granular cell tumors metastasize to lung, bone, liver and other organs over a number of years (26).
Perineurioma
A rare case of mediastinal perineurioma has been reported (58), in an endobronchial location. Perineuriomas are also subtyped, but in the mediastinum, they resemble the soft tissue perineurioma. Grossly, the cut surface of perineuriomas are white to gray, and they are well circumscribed but unencapsulated, with a firm texture. Histologically, soft tissue perineuriomas are composed of elongated neoplastic cells with wavy-shaped nuclei with eosinophilic cytoplasm and indistinct cell boundaries. Characteristically, the spindle cells have elongated nuclei and elongated and extremely thin, widely separated bipolar cytoplasmic processes. Architecturally, the cells may be seen in storiform, lamellar arrangements, or perivascular whorling formations. The stroma can vary from a more collagenous to a more myxoid or hypocellular appearance and a collagenous-myxoid background. The differential diagnosis in the mediastinum includes solitary fibrous tumor, low grade fibromyxoid sarcoma and other low-grade spindle cell neoplasms. By immunohistochemistry, perineuriomas are positive for epithelial membrane antigen and Claudin-4, and unlike other benign peripheral nerve sheath tumors, lack expression of S100 and SOX10. In general, perineuriomas are clinically benign and treated by excision.
Extracranial meningioma
Rare examples of extracranial meningiomas arising in the mediastinum or extending into the thorax from paraspinal or intracranial lesions have been described (59-64).
Malignant tumors of peripheral nerve origin
Malignant peripheral nerve sheath tumor
MPNST’s are sarcomas that can be diagnosed in the presence of a tumor that either arises from a peripheral nerve or a pre-existent neurofibroma and/or display pathologic features of Schwannian differentiation. About 25–50% of MPNST develop in patients with NF1 (65). The sarcomas develop in 10–30% of patients with plexiform neurofibromas, usually after a 10–20 years latency period. Radiation exposure, including radiation therapy is also a predisposing factor to development of MPNST.
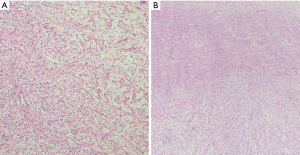
Anatomically, MPNST has been reported in all areas of the mediastinum. Grossly, MPNST appear as large, fusiform tumors that arise from a major nerve and usually measure >5 cm in diameter (48,66,67). Histologically, MPNST has a variety of appearances; however, classically, they are composed of spindle cells with vague nuclear palisading recapitulating features characteristic of Schwann cells, with wavy, or comma-shaped nuclei and amphophilic cytoplasm (Figure 9). The tumor cells are arranged in fascicles that can vary in cellularity from hypocellular and myxoid areas to cellular areas resembling adult type fibrosarcomas or synovial sarcomas (Figures 10A,B,11A,B). Proliferation of the malignant spindle cells into the perineurium or the subendothelial areas of vessels are characteristic of MPNST. Distinctive features of MPNST includes hyaline bands and nodules that resemble giant rosettes. The tumors can also exhibit heterologous elements with islands of mature cartilage or bone, mucin-secreting glands, and squamous epithelium, rhabdomyosarcomatous or angiosarcomatous elements (64). Histologically, MPNST range from low-grade lesions that can be difficult to distinguish from ANNUBP, as described above, to anaplastic tumors exhibiting a markedly pleomorphic clearly sarcomatous appearance. A small subset (5%) of MPNST’s show epithelioid morphology (EMPNST), which raises a distinctive differential diagnosis separate from that of the classic or usual spindle cell MPNST. The differential diagnosis in EMPNST includes melanoma, carcinoma, myoepithelioma, extraskeletal myxoid chondrosarcoma and other sarcomas, including some small round cell sarcomas. Association of EMPNST with NF1 is not as strong as in ordinary MPNST. In addition, EMPNST is a pattern of malignant transformation in schwannomatosis.
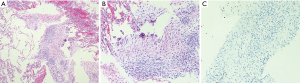
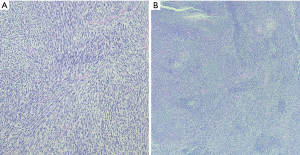
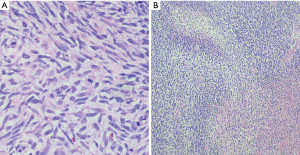
Classically, MPNST exhibits limited S100 and SOX-10 immunoreactivity and diffuse strong expression in a tumor argues against MPNST. Loss of the trimethylation marker H3k27me3 is known to be deficient in high grade MPNST, but not in low grade MPNST. Some MPNST’s show aberrant expression of desmin, myogenin and MyoD1 (Figures 9C,12A,B). Loss of neurofibromin expression has been reported in the majority of MPNST associated with NF1 . In contrast EMPNST demonstrates strong diffuse SOX10 and S100 protein expression. Although it retains expression of H3K27me3, there is frequently loss of SMARCB1. In general, keratin expression is negative in MPNST and EMPNST. In general, MPNST are resistant to chemotherapy and radioresistant. They are highly malignant tumors that are treated with surgical excision (65,68).
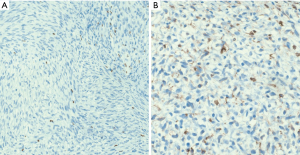
Malignant neuromuscular choristoma (malignant neuromuscular hamartoma (malignant triton tumor) (64)
Malignant neuromuscular choristomas, also reported as malignant neuromuscular hamartomas or malignant Triton tumors, are rare sarcomas that appear as well-circumscribed lesions composed of MPNST elements admixed with skeletal muscle fibers. A few cases have been described in the posterior mediastinum and the anterior mediastinum of adults in their 3rd to 5th decade of life (69-71). Patients have a poor prognosis after treatment with surgical resection and/or chemotherapy and tend to recur locally and/or develop lung metastases (68).
Extraspinal ependymoma
Rare examples of ependymomas involving the posterior mediastinum have been described (72-76). However, we have not seen any in our practice.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Saul Suster and David Suster) for the series “Mediastinal Sarcomas” published in Mediastinum. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-43). The series “Mediastinal Sarcomas” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mlika M, Marghli A, Souilem I, et al. A single-institution experience of neurogenic tumors of the mediastinum. Asian Cardiovasc Thorac Ann 2019;27:661-9. [Crossref] [PubMed]
- Topcu S, Alper A, Gulhan E, et al. Neurogenic tumours of the mediastinum: a report of 60 cases. Can Respir J 2000;7:261-5. [Crossref] [PubMed]
- Inci I, Turgut M. Neurogenic tumors of the mediastinum in children. Childs Nerv Syst 1999;15:372-6. [Crossref] [PubMed]
- Saenz NC. Posterior mediastinal neurogenic tumors in infants and children. Semin Pediatr Surg 1999;8:78-84. [Crossref] [PubMed]
- Zhu W, Chen D. Vagus nerve schwannoma in the right upper mediastinum. Thorac Cancer 2017;8:698-702. [Crossref] [PubMed]
- Fujita K, Nakashima K, Kumakura H, et al. A recurrent vagal schwannoma in the middle mediastinum after surgical enucleation. Ann Thorac Cardiovasc Surg 2014;20:832-5. [Crossref] [PubMed]
- Huang TW, Yang MH, Cheng YL, et al. Vagus nerve schwannoma in the middle mediastinum. Thorac Cardiovasc Surg 2010;58:312-4. [Crossref] [PubMed]
- Sasaki K, Kohno T, Mun M, et al. Thoracoscopic removal of middle mediastinal schwannoma originating from recurrent nerve. Thorac Cardiovasc Surg 2008;56:375-7. [Crossref] [PubMed]
- Wang W, Cui M, Ma HX, et al. A large schwannoma of the middle mediastinum: A case report and review of the literature. Oncol Lett 2016;11:1719-21. [Crossref] [PubMed]
- Vaish AK, Verma SK, Shakya S, et al. Schwannoma in anterior mediastinum with massive pericardial effusion. BMJ Case Rep 2012;2012:bcr2012007867 [Crossref] [PubMed]
- Tajima H, Tajima N, Yamamoto K, et al. Anterior mediastinal schwannoma: a case report. Radiat Med 1995;13:175-7. [PubMed]
- Tebow LE, Brown RB. Neurogenic tumors of the anterior and middle mediastinum; a report of two cases. Am Surg 1953;19:491-7. [PubMed]
- Fraga JC, Aydogdu B, Aufieri R, et al. Surgical treatment for pediatric mediastinal neurogenic tumors. Ann Thorac Surg 2010;90:413-8. [Crossref] [PubMed]
- Chen X, Ma Q, Wang S, et al. Surgical treatment of posterior mediastinal neurogenic tumors. J Surg Oncol 2019;119:807-13. [Crossref] [PubMed]
- Song YJ, Seol SH, Kim S, et al. Benign posterior mediastinal schwannoma-Multiple diagnostic imaging modalities. Clin Case Rep 2019;7:2585-7. [Crossref] [PubMed]
- Nosrati R, Anissian D, Ramezani F, et al. Benign schwannoma of posterior mediastinum accompanied by bloody pleural effusion misdiagnosed as solitary fibrous tumor: A case report. Caspian J Intern Med 2019;10:468-71. [PubMed]
- Nakashima C, Harada H, Shibata S. Mediastinal Ancient Schwannoma Causing Intrathoracic Bleeding. Ann Thorac Cardiovasc Surg 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Kang LH, Shin DH, Yoon SH. Schwannoma arising in mediastinal lymph node diagnosed by endobronchial ultrasound. Respirol Case Rep 2019;7:e00481 [Crossref] [PubMed]
- Shanmugasundaram G, Thangavel P, Venkataraman B, et al. Incidental ancient schwannoma of the posterior mediastinum in a young male: a rare scenario. BMJ Case Rep 2019;12:e227497 [Crossref] [PubMed]
- Erickson LA. Posterior Mediastinal Schwannoma (Neurilemmoma). Mayo Clin Proc 2019;94:559-60. [Crossref] [PubMed]
- Loftus TJ, Pipkin M, Machuca T, et al. Angiographic embolization followed by piecemeal resection of giant posterior mediastinal schwannoma: Case report and concise review. Int J Surg Case Rep 2018;53:250-3. [Crossref] [PubMed]
- Demiroz SM, Findik G, Aydogdu K, et al. Mediastinal epithelioid angiosarcoma arising in schwannoma: The first case in the literature. Turk Gogus Kalp Damar Cerrahisi Derg 2018;26:305-8. [Crossref] [PubMed]
- Singh A, Pattabhiraman VR, Srinivasan A, et al. Dumbbell posterior mediastinal schwannoma invading trachea: Multidisciplinary management - weight off the chest. Lung India 2018;35:269-72. [Crossref] [PubMed]
- Dy P, Lajom C, Sanchez J. Middle mediastinal schwannoma concealed by asthma and GORD. BMJ Case Rep 2018;2018:bcr2017223795 [Crossref] [PubMed]
- Sugio K, Inoue T, Inoue K, et al. Neurogenic tumors of the mediastinum originated from the vagus nerve. Eur J Surg Oncol 1995;21:214-6. [Crossref] [PubMed]
- Goldblum JW, SW, Folpe, AL. Benign tumors of peripheral nerves. In: oldblum JW, SW, Folpe, AL, editor. Enzinger and Weiss's Soft Tissue Tumors. 7th ed.: Elsevier; 2018:885-958.
- Kapoor A, Singhal MK, Narayan S, et al. Mediastinal schwannoma: A clinical, pathologic, and imaging review. South Asian J Cancer 2015;4:104-5. [Crossref] [PubMed]
- Song SH, Sim GA, Baek SH, et al. Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH) Associated with Mediastinal Schwannoma. Electrolyte Blood Press 2017;15:42-6. [Crossref] [PubMed]
- Das A, Choudhury S, Basuthakur S, et al. Massive hemoptysis: a rare presentation of posterior mediastinal, giant, benign vagal schwannoma. Arch Iran Med 2014;17:779-82. [PubMed]
- Ishibashi H, Takahashi K, Kumazawa S, et al. Successful excision of a giant mediastinal vagal schwannoma causing severe tracheal stenosis through a median sternotomy. Ann Thorac Surg 2014;98:336-8. [Crossref] [PubMed]
- Smith EE, Novalija J, Tisol WB, et al. Gradual development of unilateral Horner's syndrome in an otherwise asymptomatic elderly man. Diagnosis: right upper posterior mediastinal thoracic schwannoma. J Cardiothorac Vasc Anesth 2009;23:115-7. [Crossref] [PubMed]
- Vijendra SS, Rao RA, Prasad V, et al. A giant vagal schwannoma with unusual extension from skull base to the mediastinum. J Cancer Res Ther 2015;11:970-3. [Crossref] [PubMed]
- Wu Y, Zhang J, Chai Y. Giant mediastinal schwannoma located in the lower right side of the chest. Niger J Clin Pract 2016;19:678-80. [Crossref] [PubMed]
- Wang J, Yan J, Ren S, et al. Giant neurogenic tumors of mediastinum: report of two cases and literature review. Chin J Cancer Res 2013;25:259-62. [PubMed]
- Quartey B, Lenert J, Deb SJ, et al. Giant Posterior Mediastinal Ancient Schwannoma Requiring Thoracoabdominal Resection: A Case Report and Literature Review. World J Oncol 2011;2:191-4. [PubMed]
- Kara M, Ozkan M, Sak SD, et al. Giant ancient schwannoma of the posterior mediastinum cytologically misdiagnosed as a malignant tumour. A case report. Acta Chir Belg 2002;102:464-6. [Crossref] [PubMed]
- Prieto-Rodriguez M, Camanas-Sanz A, Bas T, et al. Psammomatous melanotic schwannoma localized in the mediastinum: diagnosis by fine-needle aspiration cytology. Diagn Cytopathol 1998;19:298-302. [Crossref] [PubMed]
- Zierold D, Halow KD. Thoracoscopic resection as the preferred approach to posterior mediastinal neurogenic tumors. Surg Laparosc Endosc Percutan Tech 2000;10:222-5. [Crossref] [PubMed]
- Fraga JC, Rothenberg S, Kiely E, et al. Video-assisted thoracic surgery resection for pediatric mediastinal neurogenic tumors. J Pediatr Surg 2012;47:1349-53. [Crossref] [PubMed]
- Nio M, Nakamura M, Yoshida S, et al. Thoracoscopic removal of neurogenic mediastinal tumors in children. J Laparoendosc Adv Surg Tech A 2005;15:80-3. [Crossref] [PubMed]
- Lee YH, Shieh SJ. Concomitant mediastinal ganglioneuroma and sciatic neurofibroma in a patient with neurofibromatosis. J Plast Reconstr Aesthet Surg 2009;62:e645-7. [Crossref] [PubMed]
- Pascoe HM, Antippa P, Irving L, et al. Rare manifestation of Neurofibromatosis type 1: A plexiform neurofibroma involving the mediastinum and lungs with endobronchial neurofibromata. J Med Imaging Radiat Oncol 2019;63:76-8. [Crossref] [PubMed]
- Jeong SC, Kim JJ, Choi SY, et al. Successful surgical treatment of massive spontaneous hemothorax due to intrathoracic secondary degeneration of a neurofibroma from mediastinal involvement of type 1 neurofibromatosis. J Thorac Dis 2018;10:E203-6. [Crossref] [PubMed]
- Okamoto J, Kubokura H, Ishii H, et al. Mediastinal Neurofibroma Originating from the Pulmonary Branch of the Right Vagus Nerve in a Patient without von Recklinghausen Disease. Thorac Cardiovasc Surg Rep 2013;2:29-31. [Crossref] [PubMed]
- Kanzaki R, Inoue M, Minami M, et al. Bilateral mediastinal neurofibroma of the vagus nerves in a patient with neurofibromatosis type 1. Ann Thorac Cardiovasc Surg 2013;19:293-6. [Crossref] [PubMed]
- Puppel ID. Neurofibroma of the posterior mediastinum. Surgery 1947;21:875-80. [PubMed]
- Al-Hajjaj MS. Bronchiectasis and mediastinal neurofibroma in a Saudi female with Ehlers-Danlos syndrome. Ann Saudi Med 2000;20:419-20. [Crossref] [PubMed]
- Kawachi R, Takei H, Furuyashiki G, et al. A malignant peripheral nerve sheath tumor of the mediastinum in a patient with neurofibromatosis type 1: report of a case. Surg Today 2008;38:945-7. [Crossref] [PubMed]
- Miettinen MM, Antonescu CR, Fletcher CDM, et al. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum Pathol 2017;67:1-10. [Crossref] [PubMed]
- D'Hulst L, Deroose C, Strybol D, et al. 18F-FDG PET/CT and MRI of a Mediastinal Malignant Granular Cell Tumor With Associated Recurrent Pericarditis. Clin Nucl Med 2018;43:589-90. [Crossref] [PubMed]
- Winchester LM, Puckett Y, Greenspon J, et al. Mediastinal Granular Cell Tumor in a 16-Year-Old Boy: A Surgical and Pathologic Perspective. Pediatr Dev Pathol 2016;19:64-8. [Crossref] [PubMed]
- Shikatani Y, Okazaki M, Sakao N, et al. A Case of Mediastinal Granular Cell Tumor with Horner's Syndrome. Ann Thorac Cardiovasc Surg 2015;21:567-9. [Crossref] [PubMed]
- Kusano J, Iguchi F, Takahashi Y, et al. Neck and superior mediastinal granular cell tumor excised via a combined approach. Auris Nasus Larynx 2015;42:72-6. [Crossref] [PubMed]
- Kim DY, Jeon HW, Kim KS, et al. A rare case of mediastinal granular cell tumor. Korean J Thorac Cardiovasc Surg 2014;47:494-6. [Crossref] [PubMed]
- Soh WM, Yeong ML, Wong KP. Malignant granular cell tumour of the mediastinum. Malays J Pathol 2014;36:149-51. [PubMed]
- De Luca G, Luciano A, Benincasa G, et al. Giant malignant granular cell tumor (GCT) of the posterior mediastinum. J Thorac Oncol 2013;8:1107-8. [Crossref] [PubMed]
- Nakao M, Hishida T, Ishii G, et al. Malignant granular cell tumor of the posterior mediastinum with dissemination. Asian Cardiovasc Thorac Ann 2012;20:71-3. [Crossref] [PubMed]
- Bolan JM, Colby TV, Folpe AL. Intrathoracic peripheral nerve sheath tumors-a clinicopathological study of 75 cases. Hum Pathol 2015;46:419-25. [Crossref] [PubMed]
- Lu C, Hu X, Xu M, et al. Posterior mediastinal ectopic meningioma: a case report. World J Surg Oncol 2015;13:156. [Crossref] [PubMed]
- Dahdal S, Andres RH, Hewer E, et al. A rare case of a large spinal meningioma with mediastinal extension and malignant behavior classified histologically as benign. Recent Results Cancer Res 2013;194:443-55. [Crossref] [PubMed]
- Mogi A, Hirato J, Kosaka T, et al. Primary mediastinal atypical meningioma: report of a case and literature review. World J Surg Oncol 2012;10:17. [Crossref] [PubMed]
- Behbahani M, Ahmad FU, Siddiqui MA, et al. Transjugular extension of meningioma into the mediastinum-a case report. Acta Neurochir (Wien) 2010;152:151-4. [Crossref] [PubMed]
- Chen F, Zhang S. Diagnosis and treatment of the primary malignant meningioma in mediastinum: a case report. South Med J 2009;102:1164-6. [Crossref] [PubMed]
- Yang X, Gao X, Wang S. Primary mediastinal malignant meningioma. Eur J Cardiothorac Surg 2009;36:217-8. [Crossref] [PubMed]
- Goldblum JW, SW, Folpe, AL. Malignant Peripheral Nerve Sheath Tumors. In: Goldblum JW, SW, Folpe, AL, editor. Enzinger and Weiss's Soft Tissue Tumors. 7th ed.: Elsevier; 2018:959-90.
- Kalra B, Kingsley PA, Bedi HS, et al. Malignant peripheral nerve sheath tumor of the anterior mediastinum: a rare presentation. Rare Tumors 2014;6:5528. [Crossref] [PubMed]
- Shimoyama T, Yoshiya K, Yamato Y, et al. Long-term survival after removal of a malignant peripheral nerve sheath tumor originating in the anterior mediastinum. Gen Thorac Cardiovasc Surg 2009;57:310-4. [Crossref] [PubMed]
- Seno N, Fukushima T, Gomi D, et al. Successful treatment with doxorubicin and ifosfamide for mediastinal malignant peripheral nerve sheath tumor with loss of H3K27me3 expression. Thorac Cancer 2017;8:720-3. [Crossref] [PubMed]
- Chaudhry I, Algazal T, Cheema A, et al. Mediastinal malignant triton tumor: A rare case series and review of literature. Int J Surg Case Rep 2019;62:115-9. [Crossref] [PubMed]
- Ren W, Xu X, Yan J, et al. Malignant triton tumor of the anterior mediastinum: A case report. Oncol Lett 2014;7:807-10. [Crossref] [PubMed]
- Lang-Lazdunski L, Pons F, Jancovici R. Malignant "Triton" tumor of the posterior mediastinum: prolonged survival after staged resection. Ann Thorac Surg 2003;75:1645-8. [Crossref] [PubMed]
- Ye WB, Zhou JP, Xu YQ, et al. Primary mediastinal ependymoma: A case report and literature review. Medicine (Baltimore) 2019;98:e17686 [Crossref] [PubMed]
- Maeda S, Takahashi S, Koike K, et al. Primary ependymoma in the posterior mediastinum. Ann Thorac Cardiovasc Surg 2011;17:494-7. [Crossref] [PubMed]
- Mori T, Nomori H, Yoshioka M, et al. A case of primary mediastinal ependymoma. Ann Thorac Cardiovasc Surg 2009;15:332-5. [PubMed]
- Estrozi B, Queiroga E, Bacchi CE, et al. Myxopapillary ependymoma of the posterior mediastinum. Ann Diagn Pathol 2006;10:283-7. [Crossref] [PubMed]
- Satti M, Firoze M, Malaker K, et al. Mediastinal myxopapillary ependymoma primary or late metastases of paracoccygeal ependymoma: a case report. Ann Diagn Pathol 2005;9:215-8. [Crossref] [PubMed]
Cite this article as: Marchevsky AM, Balzer B. Mediastinal tumors of peripheral nerve origin (so-called neurogenic tumors). Mediastinum 2020;4:32.


