
Radiation dose for thymic tumours
Foundations of radiotherapy in thymic malignancies
Thymomas and thymic carcinomas are epithelial cell tumours arising from the thymus with an incidence of 0.10–0.18 per 100,000 persons (1). Classification of thymic tumours is primarily guided by the Masaoka-Koga (MK) staging system based on the anatomic extent of disease (2). More recently, a new Tumor-Node-Metastases staging system has been developed as a collaborative effort between the International Association for the study of Lung Cancer and the International Thymic Malignancy Interest Group (3). This new TNM system was validated in a retrospective study of 76 patients showing the implications for guiding treatment indication, stage-adapted therapy, and prediction of prognosis for overall and recurrence-free survival (4). Thymic tumours are primarily treated with surgical resection, the goal of which is to remove the tumor in its entirety. Due to the anatomical complexity of the mediastinum, this goal is not always feasible. Local relapses after surgery, although rare, are frequently incurable. Thus, Post-Operative Radiotherapy (PORT) may be used with the intent of improving local control while being judicious of toxicity in the setting of prolonged clinical trajectories and proximity to critical structures. Late toxicity of radiotherapy to the thorax is dependent on the radiotherapy dose and volume of structures that are irradiated. Organs at risk in the thorax include cardiac structures, aerodigestive tract, lung parenchyma and spine. In particular, the rare but devastating occurrence of radiation induced second malignancy should be noted (5,6). A thorough understanding of these late toxicities is essential in order to facilitate an evidence-based discussion regarding the use of PORT in patients diagnosed with thymic malignancies.
Due to the rarity of this tumour, large scale randomized studies to confirm optimal treatment regimens are generally infeasible. As such, there is a paucity of literature surrounding the use of PORT and the optimal dose has not yet been established. Current NCCN guidelines suggest a PORT dose range of 45–50 gray (Gy) for completely resected disease (R0), 54 Gy for patients with microscopic residual disease (R1) and 60–70 Gy for patients with gross residual disease (R2) with the caveat that minimal evidence is available to support these recommendations (7). This review aims to summarize the current literature regarding radiotherapy indications and to explore issues surrounding radiotherapy dose-response-relationships for thymoma and thymic carcinoma.
Early reports regarding the role of PORT following visibly complete resections of invasive (MK stage II or III) thymoma provided a foundation upon which thymomas were treated for decades. A collected series of small, single institution studies of patients with completed resected MK stage II/III thymoma treated prior to 1990 found 5% versus 28% of patients developed thoracic failure with or without radiotherapy for invasive thymoma (8). Subsequently, a large retrospective clinical and pathological review of 117 patients explored the role for mediastinal irradiation following complete or incomplete surgical resection (7). Within the 99 patients with a histologic diagnosis of thymoma after pathology review, the authors identified clinical and pathological factors associated with the risk of local recurrence including MK Stage II/III, incomplete resection status and omission of PORT. When looking at the subgroup of patients with MK stage II/III thymoma that had undergone complete resection, they reported a 5-year actuarial mediastinal relapse rate of 0% in patients that received PORT versus 53% in patients that did not receive PORT (P=0.12). Although not statistically significant, likely due to the small number of events, this work highlighted a potential difference in local recurrence between patients that did and did not receive PORT. Notably, of the 8 patients with local relapse, only 3 could be successfully salvaged, highlighting the importance of local control (8). Within the subgroup of patients with MK stage III thymoma that did not have a complete resection, 13 patients had biopsy alone and 15 patients had a subtotal resection. The 5-year actuarial LRR rate was 21% with no difference in the local relapse rate, overall relapse rate or survival between irradiated patients that underwent biopsy versus subtotal resection. The authors provided some guidance regarding de facto radiation dose regimens and target volumes but did not question if these were optimal. Of the 26 patients that received radiotherapy in this analysis, the median dose was 50 Gy with a range of 32–60 Gy (22 patients received doses between 44–51.4 Gy). The results of this study support the omission of PORT for MK stage I disease and the use of PORT for completely resected MK stage II/III disease. They also suggest a role of radiotherapy for unresectable or partially resectable disease. This high-quality early analysis defined the Western approach for PORT in thymoma and laid the groundwork for ongoing studies.
Another early retrospective analysis performed in Japan looked at the use of PORT in 141 patients diagnosed with MK stage I-IV thymoma from 1957 to 1985. PORT was performed in 73.1% of patients. Radiotherapy doses ranged from 30–40 Gy for patients after complete resection and 50 Gy for those with residual disease or biopsy only. The rate of death was 5.3% after a complete resection, 43.8% after a subtotal resection and 83.3% after a biopsy only (P<0.01). Within the subgroup of completely resected thymoma, there was no significant difference in the 15-year survival rate in patients with MK stage III disease (all of whom received PORT) when compared to patients with MK stage I disease (94.7% versus 85.7% respectively, P value not reported) (9). The study investigators noted that the use of a polytetrafluoroethylene graft with ring would enable en-bloc resection of the tumour in cases with superior vena cava invasion. Thus, this work defined an alternative approach to locally advanced thymoma in which PORT was recommended for all stages of disease (although the utility in stage I patients was questioned) and identified the importance of an advanced surgical technique to obtain a complete resection in locally advanced disease. These analyses provided the initial rationale for the use of radiotherapy in thymoma and helped to guide the management of this disease for many years.
Modern evidence supporting radiation efficacy in thymoma
While these foundational studies helped to guide the initial use of PORT for thymoma, further information was required to understand the benefit of radiotherapy with respect to clinical and pathologic factors in the setting of modern surgical and radiotherapy approaches. A contemporary multi-institutional study assessed 1,320 patients treated from 1990 to 1994 across 115 institutions (10). For patients with MK stage II thymoma, 247 (100%) underwent complete resection. The 5-year local recurrence rate in patients with completely resected MK stage II disease was 1.6% for the 122 patients treated with surgery alone and 0% for the 86 patients treated with surgery and PORT. For patients with MK stage III thymoma, 170 (84.6%) underwent total resection while 18 (9.0%) underwent subtotal resection. The 5-year local recurrence rate was 3.1% for the 31 patients treated with surgery alone and 5.1% for the 78 patients treated with surgery and PORT. There was no significant difference in 10-year survival rates between completely resected patients treated with radiotherapy (including PORT) versus surgery alone (77.9% and 95.0%, respectively). Thus, this analysis did not find a statistically significant benefit of PORT compared to resection alone in thymoma. The small sample size and lack of multivariable analyses should be noted. The authors concluded that PORT was of no benefit but acknowledged the limitations of this study and stated that no conclusions could be drawn regarding the effect of radiotherapy.
An analysis from the Surveillance, Epidemiology and End Results Database was performed from 2000–2010 including 529 patients with MK stage I-IV thymoma of which 345 (65%) received PORT. The authors used propensity matching with clinical and pathological factors (but did not include comorbidity) to partially address the typical selection bias seen in PORT studies (11). Multivariable analyses in the matched population identified factors significant for overall survival including age ≥57 years, primary tumour extension, and lack of PORT receipt. Factors significant for disease specific survival included primary tumour extension and lack of PORT. There was a significant improvement in overall survival (P=0.008) and disease specific survival (P=0.008) for patients that received PORT versus no PORT. By MK stage, there was a significant improvement in overall survival for patients receiving PORT versus no PORT in stage III (P=0.049) and IV (P=0.005) disease, but not for those with stage IIB (P=0.738). There was a significant improvement in disease specific survival for patients receiving PORT versus no PORT in stage III (P=0.012) disease, but not for those with stage IIB (P=0.405) or IV (P=0.139) disease. The authors concluded that PORT was associated with improved survival in Stage III/IV thymoma and improved disease specific survival in Stage III.
An analysis of the International Thymic Malignancy Interest Group database assessed the survival benefit of PORT for completely resected MK stage II and III thymomas (12). Of 1,263 patients in this study, 689 (55%) received PORT. Patients that received PORT were younger (median age 51 versus 59), more likely to be male (60.1% versus 39.9%), have myasthenia gravis (65.7% versus 34.3%), have stage III disease (68.1% versus 31.8%), have a high histological subtype (B1/B2/B3) (61.2% versus 38.8%) and to have received chemotherapy (67.8% versus 32.2%). The use of PORT was associated with a significant improvement in overall survival in patients with MK stage II/III thymoma (P=0.002), and in the subgroups of patients with MK stage II thymoma (P=0.021), and MK stage III thymoma (P=0.0005). This analysis provides the strongest evidence to date of the benefits of PORT for MK stage II/III thymoma. Recurrence free survival was not impacted in this study although local and distant or regional relapse were not analyzed separately. Taken together, these studies indicate a benefit of PORT for completely resected MK stage II/III thymomas and suggest a potential benefit in MK stage IV disease. Again, these studies were unable to address the optimal dose of radiotherapy.
Modern evidence supporting radiation efficacy in thymic carcinoma
Thymic carcinomas constitute a distinct clinical entity which is associated with an increased risk of recurrence and a decreased rate of survival (9). Due to this aggressive clinical trajectory, the use of PORT in the setting of thymic carcinomas has been specifically assessed. Within the above mentioned multi-institutional study of 1,320 patients, there were 182 cases of thymic carcinoma. Of these, 92 (50.6%) underwent complete resection, 37 (20.3%) underwent subtotal resection, and 53 (29.18%) patients were inoperable with an overall local recurrence rate of 51.2% (10). here was no significant difference in 5-year survival rates between patients that received radiotherapy (including PORT) versus those that were treated with surgery alone (73.6% and 72.2% respectively). The authors concluded that PORT did not improve overall survival compared to surgery in this study with the caveat that no multivariable analyses were performed and the sample size was limited.
Conversely, a study from the Surveillance, Epidemiology and End Results Database was conducted from 2004–2013 looking at 312 patients with thymic carcinoma (184 of whom received PORT). The authors used a propensity matched approach which improved standardized differences in all baseline variables (13). This study found a significant improvement in overall survival for patients that received PORT versus no PORT (P=0.007). They identified a survival benefit of PORT in patients with MK stage III disease (HR 0.31; CI: 0.15–0.66) and those with local excision or partial lesion removal (HR 0.44; CI: 0.22–0.86). On multivariable analysis for prognostic factors in the matched population, the use of PORT was significantly associated with an improvement in overall survival (P=0.013).
A combined analysis of the International Thymic Malignancy Interest Group and the European Society of Thoracic Surgeons databases looked at 1,042 patients with thymic carcinoma and found a significant overall survival benefit with the use of PORT (14). Radiotherapy data were available for 754 patients of which 449 patients (60%) received PORT including 42% of those with stage I disease, 65% with stage II disease, 67% with stage III disease, 52% with stage IVA disease and 50% with stage IVB disease. On multivariable analysis, the use of radiation therapy (including PORT) was significantly associated with an improvement in overall survival (P=0.0055) and recurrence free survival (P=0.0090). They concluded that adjuvant radiotherapy is often used in patients with thymic carcinoma and seems to be associated with a significant improvement in outcomes within this retrospective study in which subgroup analyses were infeasible. These studies suggest that there may be a survival benefit with the use of PORT in patients with thymic carcinoma. Further study is required to identify patients’ subsets that may benefit most from this approach.
Selected series evaluating dose response
Collectively, the results of the above analyses provided valuable information regarding patient selection for PORT. However, there continues to be uncertainty regarding optimal radiotherapy dose regimens and whether a dose response relationship exists with respect to clinical and pathologic factors for thymoma or thymic carcinoma. Studies to date have found conflicting results. A long-term clinico-pathological study of 93 patients with MK stage I-IV thymoma treated between 1966–2004 with a median follow-up of 9.8 years attempted to identify a dose response relationship (15). This study included 14 patients with thymic carcinoma. Surgical information was available for 38 patients (41%), with complete resection documented in 14 patients. PORT was administered in 27 patients (29%) with a median dose of 50.8 Gy (range, 38.5–57.6 Gy). Radiotherapy doses ≥50 Gy were associated with improved disease-free survival (P=0.0025) and overall survival (P<0.005). These results suggest that a dose relationship may exist but are challenging to interpret given the heterogeneity in terms of resection status and histology. When looking at the role of histology in guiding treatment, the authors used three WHO-histology based groups. They found that histology was prognostic for disease free survival and overall survival. Thus, they conclude that WHO type A, AB, and B1 thymoma constitute a particularly low risk group for which the omission of adjuvant radiotherapy could be considered. This study provides valuable insights regarding the role of histological subtypes. However, while this factor should be noted, further evidence is required to understand the appropriate implementation of this factor in the clinical decision making for patients diagnosed with thymoma.
A retrospective study of 175 MK stage I-IV thymoma with a median follow-up of 54.7 months attempted to identify a dose response (16). The MK stage composition of this cohort was 47 (26.9%) MK I, 41 (23.4%) MK II, 41 (23.4%) MK III, 32 (18.3%), MK IVA, 9 (5.1%) MK IVB and 5 (2.9%) unclassified. Complete resection was achieved in 126 (72%) of patients. A total of 169 patients (96.6%) received radiotherapy. The radiotherapy dose range was 45–65 Gy. The 5-year local control rates for the 39 patients receiving ≤50 Gy and 89 patients receiving >50 Gy were 71.8% and 65.1%, respectively (P=0.200). The 5-year survival rates for patients receiving ≤50 Gy and those receiving >50 Gy were 69.8% and 81.5% respectively (P=0.020). Thus, no dose response was observed in this study; however, this analysis included a significant number of MK stage I patients and also those with incompletely resected disease.
Similarly, a retrospective single-institution study of 65 patients with completely resected MK stage III thymoma and a median follow-up of 50 months was unable to elucidate a radiotherapy dose-response relationship in the 51 patients for whom radiotherapy dose data were available (17). Radiation doses ranged from 28–60 Gy with a median dose of 56 Gy (3 patients discontinued treatment prior to 30 Gy due to toxicity). A total of 20 patients received doses ≤50 Gy while 31 patients received doses >50 Gy with 1 in-field recurrence in each group. There was no significant difference in the 10-year overall survival rates for patients receiving ≤50 Gy versus >50 Gy (65% versus 58.2% respectively, P=0.7). Similarly, there was no significant difference in the 10-year disease free survival rates for patients receiving ≤50 Gy versus >50 Gy (46.2% versus 54.9% respectively, P=0.67). Thus, this study was unable to identify a dose response relationship within this small, diverse cohort of patients.
Use of lower radiotherapy doses
The results of these studies are difficult to synthesize given the limited statistical power and heterogeneity of patients in terms of MK stage, histology and completeness of resection. This is further compounded by the uncertainty surrounding the dose threshold that should be assessed. Two of the three studies above were unable to identify a dose-response relationship using a threshold of 50 Gy. Lower dose thresholds have also been explored, given the potential clinical benefit of decreasing long-term toxicity. A multi-institutional retrospective review of 103 patients that underwent postoperative radiotherapy for completely resected thymoma included patients with MK stage I-III disease treated between 1979 to 1998 with a median follow-up of 112 months. The radiotherapy dose range was 30–61 Gy with a median radiotherapy dose of 40 Gy. A total of 39 patients received a radiotherapy dose <40 Gy, 45 patients received 40 Gy and 19 patients received doses >40 Gy. There were zero in-field recurrences regardless of radiotherapy dose provided. Despite the limitations of this small retrospective analysis which did not assess radiotherapy dose in relation to MK stage, these results demonstrated acceptable recurrence rates suggesting the potential of using lower radiotherapy doses as an effective approach for the management of thymoma (18).
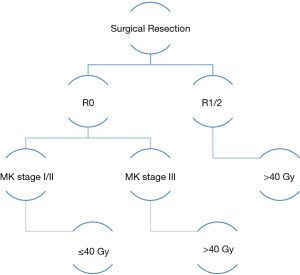
The use of lower radiotherapy doses was further explored in a retrospective single institution study of 104 patients with MK stage I-IV thymoma. After a median follow-up of 10 years, only 3 local recurrences were seen when doses ≤40 Gy were used (19). Within patients that experienced a local relapse, one patient with MK stage I disease recurred 19 years after treatment and two patients with MK stage III disease recurred within 3 years of treatment. These analyses suggest that lower radiotherapy doses may provide adequate control for early stage disease while higher doses may be appropriate for patients with increased risk (Figure 1). Further studies are required to determine the appropriate dose and whether a dose response relationship exists taking into account clinical and pathologic factors.
Conclusions
The above studies suggest a benefit in the use of PORT for patients with completely resected MK stage II/III thymoma and thymic carcinoma. The use of lower radiotherapy doses for patients with lower risk disease may provide improved therapeutic ratios and should be further explored. These analyses also highlight the challenges of identifying dose-response-relationships in patients with thymoma due to the rarity of this entity, heterogeneity of clinical and pathological factors, prolonged clinical trajectories, and low event rates. Further, the majority of patents in these studies were treated with 2D radiotherapy planning techniques and historical surgical approaches. As such, definitive studies confirming optimal radiotherapy dose approaches in the modern era are lacking. Long-term prospective studies using image-guided radiotherapy and modern surgical techniques are required to facilitate discussions regarding the optimal radiation approach in the management of thymic tumours.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mirella Marino, Brett W. Carter) for the series “Dedicated to the 10th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2019)” published in Mediastinum. The article has undergone external peer review.
Peer Review File: Available at http://dx.doi.org/10.21037/med-20-4
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-4). The series “Dedicated to the 10th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2019)” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Engels EA, Pfeiffer RM. Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer 2003;105:546-51. [Crossref] [PubMed]
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [Crossref] [PubMed]
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S65-S72. [Crossref] [PubMed]
- Ried M, Eicher MM, Neu R, et al. Evaluation of the new TNM-staging system for thymic malignancies: impact on indication and survival. World J Surg Oncol 2017;15:214. [Crossref] [PubMed]
- Eng TY, Thomas CR Jr. Radiation therapy in the management of thymic tumors. Semin Thorac Cardiovasc Surg 2005;17:32-40. [Crossref] [PubMed]
- Pan CC, Chen PC, Wang LS, et al. Thymoma is associated with an increased risk of second malignancy. Cancer 2001;92:2406-11. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Thymomas and Thymic Carcinomas (Version 1.2020). Available online: http://www.nccn.org/professionals/physician_gls/pdf/thymic.pdf. Accessed May 1, 2020.
- Curran WJ Jr, Kornstein MJ, Brooks JJ, et al. Invasive thymoma: the role of mediastinal irradiation following complete or incomplete surgical resection. J Clin Oncol 1988;6:1722-7. [Crossref] [PubMed]
- Nakahara K, Ohno K, Hashimoto J, et al. Thymoma: results with complete resection and adjuvant postoperative irradiation in 141 consecutive patients. J Thorac Cardiovasc Surg 1988;95:1041-7. [Crossref] [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. [Crossref] [PubMed]
- Lim YJ, Kim HJ, Wu HG. Role of Postoperative Radiotherapy in Nonlocalized Thymoma: Propensity-Matched Analysis of Surveillance, Epidemiology, and End Results Database. J Thorac Oncol 2015;10:1357-63. [Crossref] [PubMed]
- Rimner A, Yao X, Huang J, et al. Postoperative Radiation Therapy Is Associated with Longer Overall Survival in Completely Resected Stage II and III Thymoma-An Analysis of the International Thymic Malignancies Interest Group Retrospective Database. J Thorac Oncol 2016;11:1785-92. [Crossref] [PubMed]
- Lim YJ, Song C, Kim JS. Improved survival with postoperative radiotherapy in thymic carcinoma: A propensity-matched analysis of Surveillance, Epidemiology, and End Results (SEER) database. Lung Cancer 2017;108:161-7. [Crossref] [PubMed]
- Ahmad U, Yao X, Detterbeck F, et al. Thymic carcinoma outcomes and prognosis: results of an international analysis. J Thorac Cardiovasc Surg 2015;149:95-100, 101.e1-2. [Crossref] [PubMed]
- Harnath T, Marx A, Ströbel P, et al. Thymoma-a clinico-pathological long-term study with emphasis on histology and adjuvant radiotherapy dose. J Thorac Oncol 2012;7:1867-71. [Crossref] [PubMed]
- Zhu G, He S, Fu X, et al. Radiotherapy and prognostic factors for thymoma: a retrospective study of 175 patients. Int J Radiat Oncol Biol Phys 2004;60:1113-9. [Crossref] [PubMed]
- Fan C, Feng Q, Chen Y, et al. Postoperative radiotherapy for completely resected Masaoka stage III thymoma: a retrospective study of 65 cases from a single institution. Radiat Oncol 2013;8:199. [Crossref] [PubMed]
- Ogawa K, Uno T, Toita T, et al. Postoperative radiotherapy for patients with completely resected thymoma: a multi-institutional, retrospective review of 103 patients. Cancer 2002;94:1405-13. [Crossref] [PubMed]
- Lalani N, Safieddine N, Hwang D, et al. Risk Adapted Radiation Therapy for Thymoma: A Single-Institution 30 Year Review. Int J Radiat Oncol Biol Phys 2015;93:SE444. [Crossref]
Cite this article as: Lalani N, Brade AM. Radiation dose for thymic tumours. Mediastinum 2020;4:35.


