
Poorly-differentiated and undifferentiated sarcomas of the mediastinum: a bag of tricks
Introduction
Differentiation versus histogenesis
Classification of connective tissue neoplasms differs from most of others in a very peculiar way. As Stout and Lattes pointed out in their pioneering work, the histogenetic theory is of limited help in soft tissue pathology and the concept of differentiation is the key for understanding the classification (1). For instance—as these authors pointed out—rhabdomyosarcomas can arise in viscera, where skeletal muscle is absent; the same concept is also heralded by more recent sarcoma pathologists: indeed Professor Fletcher, in his book of oncological pathology, reports the example of liposarcoma arising in skeletal muscle (2). The classification of connective tissue neoplasms considers tumors showing a specific differentiation (i.e., Adipocytic tumors) and tumors which do not. Among the latter there are several well-defined neoplasms, each with peculiar clinical presentation, morphology, immunohistochemistry and molecular features (as for Synovial sarcoma) and the so-called Undifferentiated sarcomas (i.e., tumors that show “no identifiable line of differentiation when analyzed by presently available technology”) (3). Undifferentiated sarcomas are further sub-classified by the morphology of the malignant cells in: (I) spindle cell sarcoma, (II) pleomorphic sarcoma, (III) epithelioid sarcoma, (IV) round cell sarcoma, or (V) a combination of the above. In the last WHO classification the undifferentiated small round cell sarcomas have been separated from all the other connective tissue neoplasms, because they represent a group with a specific clinical presentation and molecular alterations (3).
Undifferentiated sarcoma
Defining the unidentifiable
Given this definition, the undifferentiated sarcoma is a diagnosis of exclusion and to make the diagnosis a step-wise approach can be helpful (4). In general, one should exclude:
- non-sarcomatous neoplasms: (i) such as carcinomas, that can be primary (an anaplastic carcinoma could arise in ectopic thyroid gland in mediastinum), or metastatic (as from a pleomorphic lung carcinoma); metastasis from other cancer type such as (ii) melanoma; and localization of a (iii) hematopoietic neoplasm (such as large cell lymphoma) (see Table 1).
- Sarcomatous differentiation of non-sarcomatous neoplasms: such as a sarcomatous differentiation of a germ cell tumor, or a heterologous differentiation of an epithelial tumor.
- Benign mesenchymal neoplasms characterized by substantial pleomorphism: such as (i) schwannoma with degenerative atypia (the so called “ancient” schwannoma), (ii) symplastic leiomyoma (that can rarely occur outside the uterus), and (iii) pleomorphic lipoma.
- Dedifferentiation or malignant transformation of a mesenchymal tumors: the former often occurs in liposarcoma, chondrosarcoma, but can also be present in other neoplasms such as solitary fibrous tumor (6), the latter usually refers to the rare process of a benign–intermediate mesenchymal neoplasms that develop an overt sarcomatous area as in malignant PEComa.

Full table
Pleomorphic sarcomas of a specific histotype; among them can be identified: (i) intimal sarcoma, (ii) pleomorphic leiomyosarcoma, (iii) pleomorphic rhabdomyosarcoma, (iv) pleomorphic liposarcoma, (v) extraskeletal osteosarcoma and (vi) myxofibrosarcoma, high-grade.
Achieving a diagnosis of undifferentiated sarcoma can therefore be challenging, however it bears relevant prognostic implications (7). Clinical characteristics, morphological features, immunohistochemical stains and molecular tests, useful to narrow down the differential, will be covered in the next sections (Figure 1).
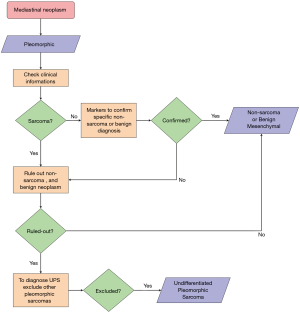
First step in the diagnosis of undifferentiated sarcomas: is it a sarcoma?
Take all the help you can get
Soft tissue books mainly focus on morphological, immunohistochemical and molecular aspects that can support the diagnosis of undifferentiated sarcoma (8-10), often—marginally—they also cover clinical features, but rarely radiologic ones are taken into consideration; this in sharp contrast with bone pathology that historically includes these features (11). Our approach is always to thoroughly revise clinical information and radiological images (a good proxy for gross examination); when neither is available, we ask. Phone calls are cheaper than immunohistochemistry and do not consume the paraffin block.
Dealing with Pandora’s box
Mediastinum, considered by Professor J. Rosai a Pandora’s Box (12), is usefully divided in four arbitrary anatomical categories: (I) superior, (II) anterior, (III) middle, and (IV) posterior. This simple framework allows to use the clinical and the radiological information more wisely: helping to prioritize the more likely differential diagnosis (13-16). Regarding mediastinal pleomorphic neoplasms, the superior mediastinum often harbors lymphomas and thyroid neoplasms. These two entities can also be found in the anterior mediastinum, where also thymic neoplasms, germ cells tumors and extra-adrenal paragangliomas develop. Lymphomas also arise in the middle mediastinum, which mostly is occupied by the heart, that can develop pleomorphic sarcomas too. Lastly, the posterior mediastinum is most often involved by mesenchymal neoplasms: the so-called “neurogenic tumors”; among these, pleomorphism—that could be worrisome on biopsies—can be seen in schwannomas, ganglioneuromas and paragangliomas. However, one should keep in mind that pleomorphism, in peripheral nerve sheath tumors, is a feature of malignancy (17). This anatomical approach is a useful point to start reasoning about the differential diagnosis.
Seeing through the box
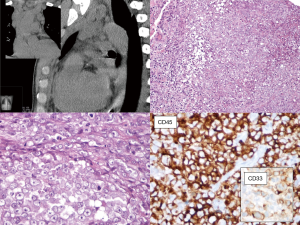
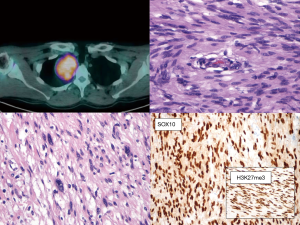
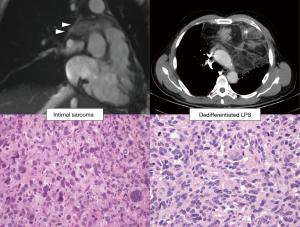
Radiology and pathology are similar disciplines and someone foresees a future fusion of the two (18); meanwhile, a good cross-talk between specialists can be very helpful for the management of the current cases and for the growth of both. In dealing with the mediastinal neoplasm, in many—if not all—instances, reviewing the imaging studies is the only way the pathologist has to reconstruct where the specimen was biopsied, or removed. The optimal setting would be a multidisciplinary discussion with a dedicated sarcoma radiologist (19,20). However, when this is not an option, direct inspection of radiological exams (even by an unexperienced pathologist) can give a lot of information on key features such as the size, the exact location (as for Figure 2), and other gross features that can be suggestive of any special diagnosis. These can be: the relationship with other structures such as vessels, nerves, bones, or other organs (as in Figures 3 and 4); the type of growth, such as a nodular expansive (as in Figure 2) or irregular and infiltrative; and other indicative features such an adipose component (as in Figure 4) or a focally necrotic process.
Always rule-out non-sarcomatous lesions first
Sarcomas represent a very rare disease (21-23): before considering them, in the mediastinum or elsewhere, pathologists should always rule-out non-sarcomatous lesions (Table 1; Figure 2). Thymic epithelial neoplasms represent the majority of lesions arising in the mediastinum; their diagnosis usually rests on the evaluation of morphological features and immunohistochemistry plays a role in the diagnosis of difficult cases, some of which may present with spindle cell or pleomorphic morphology. Rare cases of sarcomatoid thymic carcinomas may raise a differential diagnosis with mediastinal sarcomas: a positive immunostaining for cytokeratins, PAX8 and/or p63 is in keeping with a diagnosis of an epithelial tumor (24). However, it should be kept in mind that there is no specific marker able to definitively distinguish epithelial tumors, although in selected cases—such as in the differential between thymic and lung—PAX8, CD5 and CD117 might be of help (25,26). The differential diagnosis therefore rests on a meticulous clinicopathological correlation. In addition, metaplastic thymoma can be considered in the differential diagnosis of spindle cell lesions, despite lacking overt atypia. This lesion, in addition to the expression of epithelial markers, is characterized by the recently described translocation involving the Yes Associated Protein 1 (YAP1) and Mastermind Like Transcriptional Coactivator 2 (MAML2) genes (27). A sarcomatous differentiation can be observed in mediastinal germ cells tumors (28). In keeping with other germ cell tumors, also those of the mediastinum are largely characterized by the presence of isochromosome 12p and 12p copy number gain in the post pubertal setting (29). Despite never being investigated in the sarcomatoid component of mediastinal germ cell tumors, data from gonadal lesions suggest that 12p copy number gain may provide support for the diagnosis (30).
Primum non nocere
The same relative frequency approach should be kept in mind when considering benign mesenchymal tumors, indeed they tend to be much more frequent than malignant and in clinical practice they outnumber sarcomas about 100 times (31). Although specific guidelines for mediastinal sarcoma are lacking (19,20), the mainstay of therapy is radical surgery, and in other similar sites (i.e., retroperitoneum) a diagnosis of sarcoma might prompt extensive surgery with removal of other organs and structures even if not overtly infiltrated (32,33). Therefore, after the exclusion of the non-mesenchymal neoplasm, one should also exclude the benign mesenchymal one.
A frequent (pleomorphic) benign neoplasm
Schwannoma is a frequent benign mediastinal neoplasm that often shows some degree of pleomorphism (13-16). Diagnosis is usually straightforward, especially if clinical records and radiological exams report a posterior mediastinal mass, incidentally discovered, often laterally placed, that abuts the pleural surface (Figure 3). On small biopsies, two subtypes can raise concerns: (I) the ancient schwannoma, where scattered atypical pleomorphic nuclei as well as ischemic changes are present, but the Ki67 is low; (II) the cellular schwannoma—abundant in the mediastinum since often involves large nerves—is exclusively composed of hyperchromatic, tightly packed spindled cells, it can be mitotically active and have small areas of necrosis. Encapsulation and subcapsular lymphocytes are of little help on small biopsies, whereas hotspot—rather than diffuse—Ki67 labelling, a low proliferative index <20%, the retention of H3K27me3 and p16 immunoreactivity are helpful features that favor the diagnosis of cellular schwannomas in the differential with malignant peripheral nerve sheath tumor (MPNST) (34-36).
If we are sure we are dealing with a sarcoma, how to proceed?
Tricks from the bag
Subtyping of pleomorphic sarcoma has therapeutic and prognostic implications (7,32,33) and immunohistochemistry and molecular approaches to sarcoma have been systematically and extensively reviewed in several books (10,37). Herein we provide a succinct list of markers for specific sarcomas that can present with pleomorphic morphology, highlighting the criticalities of their use in this context. Moreover, these markers neither substitute reviewing clinical and radiological data, nor trump good sampling and histological evaluation. Regarding these latter aspects of sarcoma diagnosis, whenever in doubt, further sampling might provide additional morphological clues: indeed, we expect the better differentiated-component—of a dedifferentiated high-grade sarcoma—to be only focal.
Ring chromosomes ring bells
Liposarcoma is one of the most common diagnoses, and almost all the old malignant fibrous histiocytomas of the retroperitoneum (diagnostic category today replaced by undifferentiated pleomorphic sarcoma) are now diagnosed as liposarcomas (38,39). This is due to the advent of MDM2 immunohistochemistry and MDM2-amplification FISH analysis (40); they capture the underlying molecular event: a chromosomal amplification of the region 12q13-15, that can occur through several mechanisms, such as ring chromosome (41). Therefore, MDM2—and its chromosomal neighbor CDK4—expression by immunohistochemistry is often considered a good marker for the diagnosis of liposarcoma, at least in the retroperitoneum (42-45). However, in the mediastinum there is another pleomorphic sarcoma that share the same molecular alteration, and display similar morphology and immunohistochemistry: the intimal sarcoma (46). In these cases, the anatomical location is a very useful prompt for the diagnosis, and—in the mediastinum—we caution to diagnose any of these two entities in absence of further information (Figure 4).
Countermoves when the sarcoma shows the muscles (striated or smooth)
Myogenic differentiation, either toward smooth muscle or skeletal muscle is not a rare event in sarcomas (47-49). To further complicate the picture, common markers for myogenic differentiation namely desmin and α-smooth muscle actin are expressed in non-muscular neoplasms too (50-54). Therefore, when dealing with a pleomorphic sarcoma, the diagnosis of leiomyosarcoma and rhabdomyosarcoma can be tricky. Strict diagnostic criteria should be used: the former shall at least focally show the classic morphology (eosinophilic spindle cells with a vesicular cigar-shaped nucleus) and extensive positivity for α-smooth muscle actin (or calponin), or focal and strong positivity for desmin or h-caldesmon (55-57); moreover liposarcoma can show full blown smooth muscle differentiation (48,58) and therefore should be excluded. Pleomorphic rhabdomyosarcoma—that will show its rhabdomyoblastic differentiation almost exclusively via immunohistochemistry (either myogenin or MyoD1) (49,59)—should also be differentiated from those neoplasms that can have heterologous skeletal muscle differentiation such as liposarcoma and the malignant peripheral nerve sheath tumor (MPNST) (3,48). We covered the former in the previous paragraph and the latter will be covered in the next one.
Hope-loss might be more specific for MPNST than H3K27me3-loss
MPNST is notoriously one of the most difficult diagnosis in the soft tissue (3,60). This is probably caused by its definition: MPNST are either (I) malignant spindle cell tumor arising from (i) a nerve or (ii) benign peripheral nerve sheath tumor or (iii) in a patient with type 1 neurofibromatosis; or (II) tumor showing histological and immunohistochemical features suggesting Schwannian differentiation (3). This is often shown by a patchy positivity for S100 and SOX10 (61). Recently, an epigenetic feature—the histone 3 lysine 27 (H3K27) trimethylation deficit—has been associated to MPNST, and since it can be detected by immunohistochemistry (as loss H3K27me3 in neoplastic cells), many authors include this as a useful marker for MPNST diagnosis, especially in the differential with other sarcomas (62-64). Of note, this antibody can be useful in “sexing” normal tissue as it marks Barr’s body in female (65). However, since nobody is perfect, H3K27me3 does not distinguish MPNST from melanoma (66), and in this case, history, clinical presentation and molecular analysis can help (5); moreover, this “loss” staining pattern has also been described in radiation induced sarcoma (other than MPNST) (67).
A heavy bag to carry
When Pandora opened the Box all the evils came into the word (68), and carrying on the metaphor, one should—if not prepared—refrain from opening it. Expertise is central in sarcoma management and it is associated with a better prognosis and reduced costs of patient management (69,70). European guidelines endorse histologic review whenever the original diagnosis was made outside a reference center or network (19), and also this strategy has been shown to be cost effective (71,72). From a practical point of view, this means that if you do not deal with sarcoma on a daily-weekly basis, it is wise to cooperate with someone that does.
In summary, when facing a mediastinal neoplasm showing pleomorphism one shall exclude non-mesenchymal and benign mesenchymal tumors first. Moreover, specific sarcoma histotypes that can show pleomorphism should also be excluded, given the clinical and therapeutic implications. Only after all the other diagnoses have been excluded one can sign out an undifferentiated pleomorphic sarcoma. This process should always include a meticulous review of the clinical and the radiological data.
Acknowledgments
We thank clinicians and radiologists for providing accurate descriptions and clinical information.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Saul Suster and David Suster) for the series “Mediastinal Sarcomas” published in Mediastinum. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-54). The series “Mediastinal Sarcomas” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stout A, Lattes R. Tumors of the Soft Tissues. Washington, D.C.: Armed Forces Institute of Pathology; 1967.
- Fletcher CD. Diagnostic Histopathology of Tumors. 4th Edition. 2013.
- WHO Classification of Tumours Editorial Board, editor. Soft Tissue and Bone Tumours. 5th ed. World Health Organization; 2020.
- Goldblum JR. An approach to pleomorphic sarcomas: can we subclassify and does it matter? Mod Pathol 2014;27:S39-46. [Crossref] [PubMed]
- Agaimy A, Specht K, Stoehr R, et al. Metastatic Malignant Melanoma With Complete Loss of Differentiation Markers (Undifferentiated/Dedifferentiated Melanoma). Am J Surg Pathol 2016;40:181-91. [Crossref] [PubMed]
- Dagrada GP, Spagnuolo RD, Mauro V, et al. Solitary fibrous tumors: loss of chimeric protein expression and genomic instability mark dedifferentiation. Mod Pathol 2015;28:1074-83. [Crossref] [PubMed]
- Hornick JL. Subclassification of pleomorphic sarcomas: How and why should we care? Ann Diagn Pathol 2018;37:118-24. [Crossref] [PubMed]
- Montgomery EA, Ware AD, Gardner JM. Survival Guide to Soft Tissue Pathology. Innovative Pathology Press; 2019.
- Goldblum JR, Weiss SW, Folpe AL. Enzinger and Weiss’s Soft Tissue Tumors. Elsevier Health Sciences; 2013.
- Hornick JL. Practical Soft Tissue Pathology: A Diagnostic Approach. Elsevier Health Sciences; 2017.
- Ackerman LV, Spjut H. Tumors of Bone and Cartilage. Washington, D.C.: Armed Forces Institute of Pathology; 1962.
- Rosai J. Rosai and Ackerman’s Surgical Pathology. Elsevier Health Sciences; 2011.
- Blegvad S, Lippert H, Simper LB, et al. Mediastinal tumours: A report of 129 cases. Scand J Thorac Cardiovasc Surg 1990;24:39-42. [Crossref] [PubMed]
- Cohen AJ, Thompson L, Edwards FH, et al. Primary cysts and tumors of the mediastinum. Ann Thorac Surg 1991;51:378-84. [Crossref] [PubMed]
- Oldham HN Jr, Sabiston DC Jr. Primary tumors and cysts of the mediastinum. Monogr Surg Sci 1967;4:243. [PubMed]
- Ringertz N. Mediastinal tumors and cysts. J Thorac Surg 1956;31:458-87. [Crossref] [PubMed]
- Miettinen MM, Antonescu CR, Fletcher CDM, et al. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1—a consensus overview. Hum Pathol 2017;67:1-10. [Crossref] [PubMed]
- Jha S, Topol EJ. Adapting to Artificial Intelligence. JAMA 2016;316:2353. [Crossref] [PubMed]
- Casali PG, Abecassis N, Bauer S, et al. Soft tissue and visceral sarcomas: ESMO–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol 2018;29:iv51-67. [Crossref] [PubMed]
- von Mehren M, Randall RL, Benjamin RS, et al. Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:536-63. [Crossref] [PubMed]
- Gustafson P. Soft tissue sarcoma: epidemiology and prognosis in 508 patients. Acta Orthop Scand Suppl 1994;259:1-31. [Crossref] [PubMed]
- Gatta G, Van Der Zwan JM, Casali PG, et al. Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer 2011;47:2493-511. [Crossref] [PubMed]
- Gatta G, Capocaccia R, Botta L, et al. Burden and centralised treatment in Europe of rare tumours: results of RARECAREnet—a population-based study. Lancet Oncol 2017;18:1022-39. [Crossref] [PubMed]
- Weissferdt A, Moran CA. Immunohistochemistry in the diagnosis of thymic epithelial neoplasms. Appl Immunohistochem Mol Morphol 2014;22:479-87. [Crossref] [PubMed]
- Kriegsmann M, Muley T, Harms A, et al. Differential diagnostic value of CD5 and CD117 expression in thoracic tumors: A large scale study of 1465 non-small cell lung cancer cases. Diagn Pathol 2015;10:210. [Crossref] [PubMed]
- Asirvatham JR, Esposito MJ, Bhuiya TA. Role of PAX-8, CD5, and CD117 in distinguishing thymic carcinoma from poorly differentiated lung carcinoma. Appl Immunohistochem Mol Morphol 2014;22:372-6. [Crossref] [PubMed]
- Vivero M, Davineni P, Nardi V, et al. Metaplastic thymoma: a distinctive thymic neoplasm characterized by YAP1-MAML2 gene fusions. Mod Pathol 2020;33:560-5. [Crossref] [PubMed]
- Malagón HD, Valdez AMC, Moran CA, et al. Germ cell tumors with sarcomatous components: a clinicopathologic and immunohistochemical study of 46 cases. Am J Surg Pathol 2007;31:1356-62. [Crossref] [PubMed]
- Kao C-S, Bangs CD, Aldrete G, et al. A clinicopathologic and molecular analysis of 34 mediastinal germ cell tumors suggesting different modes of teratoma development. Am J Surg Pathol 2018;42:1662-73. [Crossref] [PubMed]
- Idrees MT, Ulbright TM, Epstein JI. Fluorescent in situ hybridization analysis for 12p alterations in sarcomatoid yolk sac tumors. Am J Surg Pathol 2019;43:1566-73. [Crossref] [PubMed]
- Myhre-Jensen O. A consecutive 7-year series of 1331 benign soft tissue tumours: clinicopathologic data. Comparison with sarcomas. Acta Orthop Scand 1981;52:287-93. [Crossref] [PubMed]
- Gronchi A, Miceli R, Colombo C, et al. Frontline extended surgery is associated with improved survival in retroperitoneal low-to intermediate-grade soft tissue sarcomas. Ann Oncol 2012;23:1067-73. [Crossref] [PubMed]
- Bonvalot S, Miceli R, Berselli M, et al. Aggressive surgery in retroperitoneal soft tissue sarcoma carried out at high-volume centers is safe and is associated with improved local control. Ann Surg Oncol 2010;17:1507-14. [Crossref] [PubMed]
- Pekmezci M, Reuss DE, Hirbe AC, et al. Morphologic and immunohistochemical features of malignant peripheral nerve sheath tumors and cellular schwannomas. Mod Pathol 2015;28:187-200. [Crossref] [PubMed]
- White W, Shiu MH, Rosenblum MK, et al. Cellular schwannoma. A clinicopathologic study of 57 patients and 58 tumors. Cancer 1990;66:1266-75. [Crossref] [PubMed]
- Woodruff JM, Godwin TA, Erlandson RA, et al. Cellular schwannoma: a variety of schwannoma sometimes mistaken for a malignant tumor. Am J Surg Pathol 1981;5:733-44. [Crossref] [PubMed]
- Goldblum JR, Weiss SW, Folpe AL. Enzinger and Weiss’s Soft Tissue Tumors. Elsevier Health Sciences; 2019.
- Le Guellec S, Chibon F, Ouali M, et al. Are peripheral purely undifferentiated pleomorphic sarcomas with MDM2 amplification dedifferentiated liposarcomas? Am J Surg Pathol 2014;38:293-304. [Crossref] [PubMed]
- Renne SL, Iwenofu OH. Pathology of retroperitoneal sarcomas: A brief review. J Surg Oncol 2018;117:12-24. [Crossref] [PubMed]
- Coindre JM, Mariani O, Chibon F, et al. Most malignant fibrous histiocytomas developed in the retroperitoneum are dedifferentiated liposarcomas: A review of 25 cases initially diagnosed as malignant fibrous histiocytoma. Mod Pathol 2003;16:256-62. [Crossref] [PubMed]
- Italiano A, Bianchini L, Keslair F, et al. HMGA2 is the partner of MDM2 in well-differentiated and dedifferentiated liposarcomas whereas CDK4 belongs to a distinct inconsistent amplicon. Int J Cancer 2008;122:2233-41. [Crossref] [PubMed]
- Thway K, Flora R, Shah C, et al. Diagnostic utility of p16, CDK4, and MDM2 as an immunohistochemical panel in distinguishing well-differentiated and dedifferentiated liposarcomas from other adipocytic tumors. Am J Surg Pathol 2012;36:462-9. [Crossref] [PubMed]
- Pilotti S, Torre G, Lavarino C. Distinct MDM2/p53 expression patterns in liposarcoma subgroups: implications for different pathogenetic mechanisms. J Pathol 1997;181:14-24. [Crossref] [PubMed]
- Kammerer-Jacquet SF, Thierry S, Cabillic F, et al. Differential diagnosis of atypical lipomatous tumor/well-differentiated liposarcoma and dedifferentiated liposarcoma: utility of p16 in combination with MDM2 and CDK4 immunohistochemistry. Hum Pathol 2017;59:34-40. [Crossref] [PubMed]
- Weaver J, Downs-Kelly E, Goldblum JR, et al. Fluorescence in situ hybridization for MDM2 gene amplification as a diagnostic tool in lipomatous neoplasms. Mod Pathol 2008;21:943-9. [Crossref] [PubMed]
- Neuville A, Collin F, Bruneval P, et al. Intimal Sarcoma Is the Most Frequent Primary Cardiac Sarcoma. Am J Surg Pathol 2014;38:461. [Crossref] [PubMed]
- Colombo C, Miceli R, Collini P, et al. Leiomyosarcoma and sarcoma with myogenic differentiation: Two different entities or 2 faces of the same disease? Cancer 2012;118:5349-57. [Crossref] [PubMed]
- Gronchi A, Collini P, Miceli R, et al. Myogenic Differentiation and Histologic Grading Are Major Prognostic Determinants in Retroperitoneal Liposarcoma. Am J Surg Pathol 2015;39:383-93. [Crossref] [PubMed]
- Folpe AL. MyoD1 and myogenin expression in human neoplasia: A review and update. Adv. Anat Pathol 2002;9:198-203. [Crossref] [PubMed]
- Bohman SL, Goldblum JR, Rubin BP, et al. Angiomatoid fibrous histiocytoma: an expansion of the clinical and histological spectrum. Pathology 2014;46:199-204. [Crossref] [PubMed]
- Folpe AL, Weiss SW, Fletcher CD, et al. Tenosynovial giant cell tumors: evidence for a desmin-positive dendritic cell subpopulation. Mod Pathol 1998;11:939-44. [PubMed]
- Howitt BE, Fletcher CDM. Mammary-type Myofibroblastoma: Clinicopathologic Characterization in a Series of 143 Cases. Am J Surg Pathol 2016;40:361-7. [Crossref] [PubMed]
- Nonaka D, Bishop PW. Sarcoma-like tumor of head and neck skin. Am J Surg Pathol 2014;38:956-65. [Crossref] [PubMed]
- Longacre TA, Egbert BM, Rouse RV. Desmoplastic and spindle-cell malignant melanoma. An immunohistochemical study. Am J Surg Pathol 1996;20:1489-500. [Crossref] [PubMed]
- Fletcher CD, Gustafson P, Rydholm A, et al. Clinicopathologic re-evaluation of 100 malignant fibrous histiocytomas: prognostic relevance of subclassification. J Clin Oncol 2001;19:3045-50. [Crossref] [PubMed]
- Deyrup AT, Haydon RC, Huo D, et al. Myoid differentiation and prognosis in adult pleomorphic sarcomas of the extremity: An analysis of 92 cases. Cancer 2003;98:805-13. [Crossref] [PubMed]
- Robin YM, Penel N, Pérot G, et al. Transgelin is a novel marker of smooth muscle differentiation that improves diagnostic accuracy of leiomyosarcomas: a comparative immunohistochemical reappraisal of myogenic markers in 900 soft tissue tumors. Mod Pathol 2013;26:502-10. [Crossref] [PubMed]
- Binh MBN, Guillou L, Hostein I, et al. Dedifferentiated liposarcomas with divergent myosarcomatous differentiation developed in the internal trunk: a study of 27 cases and comparison to conventional dedifferentiated liposarcomas and leiomyosarcomas. Am J Surg Pathol 2007;31:1557-66. [Crossref] [PubMed]
- Furlong MA, Mentzel T, Fanburg-Smith JC. Pleomorphic rhabdomyosarcoma in adults: a clinicopathologic study of 38 cases with emphasis on morphologic variants and recent skeletal muscle-specific markers. Mod Pathol 2001;14:595-603. [Crossref] [PubMed]
- Le Guellec S, Decouvelaere A, Filleron T, et al. Malignant Peripheral Nerve Sheath Tumor Is a Challenging Diagnosis A Systematic Pathology Review, Immunohistochemistry, and Molecular Analysis in 160 Patients From the French Sarcoma Group Database. Am J Surg Pathol 2016;40:896-908. [Crossref] [PubMed]
- Kang Y, Pekmezci M, Folpe AL, et al. Diagnostic utility of SOX10 to distinguish malignant peripheral nerve sheath tumor from synovial sarcoma, including intraneural synovial sarcoma. Mod Pathol 2014;27:55-61. [Crossref] [PubMed]
- Pekmezci M, Cuevas-Ocampo AK, Perry A, et al. Significance of H3K27me3 loss in the diagnosis of malignant peripheral nerve sheath tumors. Mod Pathol 2017;30:1710-9. [Crossref] [PubMed]
- Prieto-Granada CN, Wiesner T, Messina JL, et al. Loss of H3K27me3 Expression Is a Highly Sensitive Marker for Sporadic and Radiation-induced MPNST. Am J Surg Pathol 2016;40:479-89. [Crossref] [PubMed]
- Schaefer IM, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol 2016;29:4-13. [Crossref] [PubMed]
- Schaefer IM, Minkovsky A, Hornick JL. H3K27me3 immunohistochemistry highlights the inactivated X chromosome (Xi) and predicts sex in non-neoplastic tissues. Histopathology 2016;69:702-4. [Crossref] [PubMed]
- Le Guellec S, Macagno N, Velasco V, et al. Loss of H3K27 trimethylation is not suitable for distinguishing malignant peripheral nerve sheath tumor from melanoma: A study of 387 cases including mimicking lesions. Mod Pathol 2017;30:1677-87. [Crossref] [PubMed]
- Panse G, Mito JK, Ingram DR, et al. Radiation-Associated Sarcomas other than Malignant Peripheral Nerve Sheath Tumors Demonstrate Loss of Histone H3K27 Trimethylation. Histopathology 2020. [Epub ahead of print]. [PubMed]
- Hesiod. Work and Days. 700 BC.
- Bonvalot S, Gaignard E, Stoeckle E, et al. Survival benefit of the surgical management of retroperitoneal sarcoma in a reference center: a Nationwide Study of the French Sarcoma Group from the NetSarc Database. Ann Surg Oncol 2019;26:2286-93. [Crossref] [PubMed]
- Martin-Broto J, Hindi N, Cruz J, et al. Relevance of Reference Centers in Sarcoma Care and Quality Item Evaluation: Results from the Prospective Registry of the Spanish Group for Research in Sarcoma (GEIS). Oncologist 2019;24:e338-46. [Crossref] [PubMed]
- Perrier L, Buja A, Mastrangelo G, et al. Clinicians’ adherence versus non adherence to practice guidelines in the management of patients with sarcoma: a cost-effectiveness assessment in two European regions. BMC Health Serv Res 2012;12:82. [Crossref] [PubMed]
- Perrier L, Rascle P, Morelle M, et al. The cost-saving effect of centralized histological reviews with soft tissue and visceral sarcomas, GIST, and desmoid tumors: The experiences of the pathologists of the French Sarcoma Group. PLoS One 2018;13:e0193330. [Crossref] [PubMed]
Cite this article as: Renne SL, Di Tommaso L. Poorly-differentiated and undifferentiated sarcomas of the mediastinum: a bag of tricks. Mediastinum 2021;5:3.


