
Life-threatening massive bleeding in the pulmonary trunk adjacent to the right ventricular outflow tract during the resection of a large mediastinal germ cell tumor: proposed safety measures in the absence of cardiovascular surgeons: a case report
Introduction
We experienced a case of massive bleeding that occurred during the resection of a large primary mediastinal nonseminomatous germ cell tumor (PMNSGCT) in the pulmonary trunk adjacent to the right ventricular outflow tract in the absence of cardiovascular surgeons. PMNSGCTs are often detected after they have already grown to a considerable size, and intensive chemotherapy accompanied by subsequent resection forms the standard therapeutic strategy (1-5). In our case, the bleeding occurred accidentally as described below. We herein discuss the therapeutic strategies for extragonadal PMNSGCTs, focusing on surgical management. Furthermore, we present our consensus on how to safely perform surgeries for large thoracic tumors at our institution without the cardiovascular surgery department. This consensus emerged after discussing our experience with all the members involved in the surgery. We present the following case in accordance with CARE reporting checklist (available at http://dx.doi.org/10.21037/med-20-66).
Case presentation
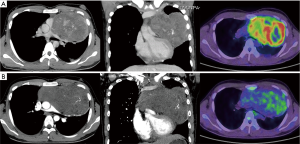
The patient was a 21-year-old male whose anterior mediastinum tumor of 14×10 cm in size was diagnosed as PMNSGCT using a computed tomography-guided needle biopsy. This tumor was a mixture of a yolk sac tumor and an immature teratoma (Figure 1A). His alpha-fetoprotein levels were approximately 10,000 ng/mL prior to treatment and normalized to 4 ng/mL after four cycles of bleomycin + etoposide + cisplatin chemotherapy. In contrast, the teratoma component of the PMNSGCT grew to a size of 16×14 cm (Figure 1B). The consequent mediastinal compression aggravated several symptoms, including the inability to remain in the supine position, resulting in growing teratoma syndrome (6). At this point, his predicted forced expiratory volume in 1 s was 50% and his Eastern Cooperative Oncology Group Performance Status score was 2. Resection was thus essential.
In general, chemotherapy for PMNSGCT causes extensive tumor necrosis that is more marked peripherally, and this oncological characteristic allows for its complete resection, even in cases that appear radiologically difficult (1,2). Surgeons deemed it possible to resect the tumor from the adjacent neighboring organs, including the ascending aorta, pulmonary trunk, and pericardium, by dissecting the peripheral necrotic tissue.
Because there is no cardiovascular surgery department at our cancer center, whenever necessary, we are assisted in daily practice by collaborating cardiovascular surgeons from another hospital, and we always strive to work closely with them. In this case, however, no cardiovascular surgeons were consulted for assistance in advance owing to the abovementioned tumor characteristics in addition to our previous experiences with PMNSGCT resections.
Safety measures, including airway management in the cephalad position and securing the femoral blood roots for assisted circulation, were taken during anesthesia (Figure 2A). Left hemi-clamshell thoracotomy (HCST) was selected. For obtaining a larger operative field than conventional procedure, we employed a modified HCST, combining full sternotomy, separation of caudal rib cartilage, large lateral fifth intercostal incision, and a supraclavicular transverse incision. The left arm was raised to expose the left side of the thorax.
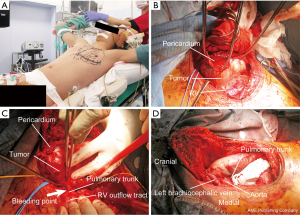
During surgery, we unexpectedly observed that the tumor extended inside and outside of the pericardium, and it appeared to have grown from the pericardium and caused cardiac compression (Figure 2B). Several frozen sections sampled from the peripheral necrotic tissue revealed no residual viable cells on the margins. As expected, the tumor was dissected from the adjacent right ventricle and ascending aorta. Subsequently, while carefully dissecting the tumor from the pulmonary trunk, the forceps held by the assistant accidentally touched the tensed pulmonary trunk adjacent to the right ventricular outflow tract, causing bleeding (Video 1). We immediately performed compression hemostasis using TachoSil® (Takeda GmbH, Linz, Austria), a collagen patch coated with human fibrinogen and thrombin, and contacted the collaborating cardiovascular surgery department at another hospital for assistance. Hemostasis was difficult to achieve; bleeding increased massively, thus causing hemorrhagic shock. Direct cardiac massage was required, and venoarterial (VA) extracorporeal membrane oxygenation (ECMO) was introduced from the inguinal route. The bleeding was treated by astriction using more TachoSil® and gauze, and VA-ECMO was established after approximately 20 min by our own team. Meanwhile, the blood flow in the fundus arteriole was narrowly maintained; however, bleeding increased via cardiac massage, and the in vivo optical spectroscopy (INVOS™) value decreased for approximately 10 min. Together with VA-ECMO establishment, provisional hemostasis was achieved. The cardiac surgeons arrived approximately 55 min after contacting them. Upon their arrival, a suction circuit was added, and hemostasis was achieved by purse string suture using 4-0 polypropylene and using a finger to control the bleeding. Thus, we were able to proceed with the resection (Figure 2C). Finally, the tumor was resected using modified HCST with combined partial resection of the pericardium and left upper lung and pericardial patch plasty (Figure 2D). The left phrenic nerve was found to be involved in the tumor, thereby necessitating its resection. The surgery lasted 8 h, and 7 L of blood was lost. Based on the above incidence, postoperative consciousness and high brain dysfunction were of utmost concern. However, the patient fully awoke on the following day without any brain dysfunction or paralysis. He was discharged 14 days after surgery. The modified HCST applied to this patient resulted in a very large wound, but it did not hinder wound healing and the postoperative course was uneventful. Pathological analysis revealed a complete resection of the PMNSGCT, which was composed of a mixture of immature teratoma and yolk sac tumor, originating from the pericardial tissue. Postoperative adjuvant chemotherapy was not considered because of the complete resection of the tumor and the physical condition of the patient after the highly invasive surgery. One year after surgery, the patient is in good condition without recurrence and his follow-up continues on an outpatient basis.
This was the first instance of establishing intraoperative emergent VA-ECMO at our cancer center without any help from cardiac surgeons. All members involved in this surgery (surgeons, anesthesiologists, operation room nursing staffs, and medical engineers) held a discussion and decided upon an in-hospital consensus so that similar surgeries could be safely performed at our institution without the cardiovascular surgery department (Table 1). Thereafter, we have been thoroughly following this consensus when performing surgeries for thoracic tumors that may require the assistance of cardiovascular surgeons. The present case was presented at the medical safety meeting at our institution and at the 73rd Annual Meeting of the Japanese Association for Thoracic Surgery in October 2020. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.

Full table
Discussion
In the present case, we should have presumed the possibility of unexpected events and consulted cardiac surgeons in advance. Moreover, their assistance should have been requested sooner, i.e., when we first observed the unexpected findings during surgery. The attending anesthesiologist (AN) secured the femoral artery and vein for access route in advance, with VA-ECMO almost being on standby as a safety measure in case of critical bleeding, and medical engineers who could handle it were also present, which enabled successful VA-ECMO establishment by ourselves without cardiac surgeons.
For controlling bleeding from the main pulmonary trunk in this case, inserting a Fogarty catheter or a urinary catheter into the bleeding site, inflating it, and pulling it back to stop the bleeding until suturing was completed could have been a possible hemostasis measure; however, this idea did not occur to us intraoperatively, and it could also have caused the tear to widen. As an alternative, another biological patch, such as Veriset™ (Medtronic), may also be effective for hemostasis of the massive bleeding; however, we have no experience of its use.
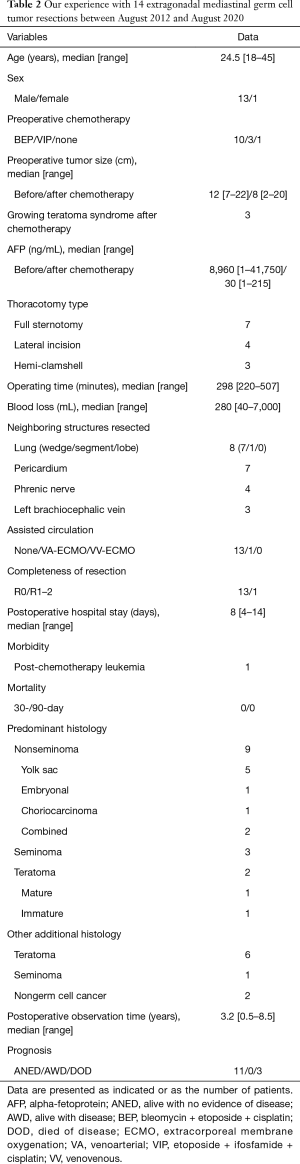
Understanding the oncological characteristics of PMNSGCTs is important when considering treatment strategies. Extragonadal primary mediastinal germ cell tumors mainly consist of teratomas, seminomatous germ cell tumors, and PMNSGCTs, and the established treatment strategies are tumor-specific (2). We have experience with 14 resections of extragonadal mediastinal germ cell tumors, including PMNSGCTs, between August 2012 and August 2020 (Table 2), and only the present case required assisted circulation. PMNSGCTs with more immature teratoma components tend to grow larger, resulting in growing teratoma syndrome after preoperative intensive chemotherapy (6). Because PMNSGCTs grow around mediastinal organs, deciding on the extent of resection and whether or not to concurrently resect adjacent organs is difficult. Kesler et al. described the strategies for surgical margins based on their extensive experience with PMNSGCT resections as follows (1): “The effectiveness of cisplatin-based chemotherapy for germ cell cancer usually results in extensive tumor necrosis being more marked peripherally. This finding usually allows a complete resection, which minimizes operative morbidity by preserving critical structures, such as lung, great veins, phrenic nerves, and cardiac chambers. Intraoperative frozen section analyses of surgical margins should be performed in cases where critical structures abutting the residual mass are preserved or visibly close margins exist”. In other words, preserving important organs that would otherwise be difficult to preserve in case of other tumors, such as lung or thymic carcinomas, is feasible; thus, we should strive to make such an effort. To date, we have been treating PMNSGCTs according to the Kesler’s strategy; based on these oncological characteristics and our previous experiences, the attending surgeon (NS) determined that the tumor in the present case was resectable at our own institution. During surgery, nearly the entire tumor was dissected at the margin of the necrotic tissue, and we reconfirmed the oncological characteristics of the PMNSGCT. It is crucial to understand the specific nature of the PMNSGCT and recognize the specific surgical strategy and technique necessary for resecting this tumor, the circumstances in which bleeding can occur, and particularly the dissection at the boundary of adjacent neighboring organs.
Full table
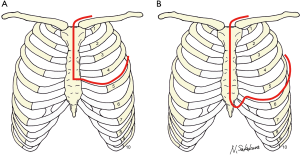
HCST (7-10) was developed from the classic and famous Masaoka’s anterior approach (11) and is generally performed using a combination of longitudinal partial sternotomy and anterolateral thoracotomy (Figure 3A). In the present case, we used an enlarged modified HCST, combining full sternotomy, separation of caudal rib cartilage, large lateral fifth intercostal incision, and a supraclavicular transverse incision (Figure 3B). The advantage of this modification is that the sternum opens wide on both sides compared with the conventional procedure, resulting in a much wider view of the mediastinum and hemithorax.
There are several important points to keep in mind when resecting a large mediastinal tumor. Regarding anesthesia (12-14), evaluating the stenosis and dilatation of the airway, the degree of blood flow disruption in the vasculature (particularly in the superior vena cava and left and right pulmonary arteries), changes in the respiratory status according to body position, the location of the infusion route, the type of anesthetic agent, and whether to employ double- or single-lumen intubation is important. Assisted circulation can be classified as VA-ECMO, which provides cardiopulmonary support, and venovenous (VV) ECMO, which provides pulmonary support. In Japan, the former is generally referred to as percutaneous cardiopulmonary support and the latter simply as ECMO. It is noted that when respiratory support is provided using VA-ECMO and pump flow is low and cardiac blood flow is maintained to some extent, insufficient oxygenated cardiac blood can flow into the coronary arteries and head, resulting in myocardial ischemia and possibly even cerebral ischemia. Therefore, monitoring cerebral ischemia is essential; using INVOS™ (noninvasive monitoring of regional cerebral oxygen saturation) is a common practice. VV-ECMO can be safer than VA-ECMO if cardiac output is preserved because partial organ ischemia is less likely to occur. VA- or VV-ECMO standby is reportedly recommended for resecting large tumors (14-16), and thus, even general thoracic surgeons should be familiar with the characteristics of assisted circulation. It is essential that the surgeon and the anesthesiologist discuss the surgery together.
Our consensus (Table 1) is only in-hospital and cannot be generalized. However, if the bleeding had not occurred, we would not have thoroughly examined the consensus. This clinical experience was extremely unusual, and we were hesitant to present it; nevertheless, it was very meaningful and resulted in a constructive consensus. We would like to share this experience with thoracic clinicians; from a medical safety viewpoint, the oncological characteristics of PMNSGCTs and the preparedness and fundamentals of the surgery for large mediastinal tumors should always be carefully considered. We hope this brief report will be of help to thoracic surgeons and all medical teams involved in such critical surgeries.
Acknowledgments
We sincerely thank the many medical staff members who worked hard to save the patient’s life during the surgery.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/med-20-66
Peer Review File: Available at http://dx.doi.org/10.21037/med-20-66
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-66). NS serves as an unpaid editorial board member of Mediastinum from Jul 2020 to Jun 2022. The other authors have no conflicts of interest to be declared.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kesler KA, Rieger KM, Hammoud ZT, et al. A 25-year single institution experience with surgery for primary mediastinal nonseminomatous germ cell tumors. Ann Thorac Surg 2008;85:371-8. [Crossref] [PubMed]
- Kesler KA. Germ cell tumors of the mediastinum. In: Pearson FG, Cooper JD, Deslauriers J, et al. editors. Pearson's thoracic and esophageal surgery. 3rd ed. Philadelphia: Churchill Livingstone, 2008;chapter 132:1615-21.
- Kesler KA, Rieger KM, Ganjoo KN, et al. Primary mediastinal nonseminomatous germ cell tumors: the influence of postchemotherapy pathology on long-term survival after surgery. J Thorac Cardiovasc Surg 1999;118:692-700. [Crossref] [PubMed]
- Wright CD, Kesler KA. Surgical techniques and outcomes for primary nonseminomatous germ cell tumors. Chest Surg Clin N Am 2002;12:707-15. [Crossref] [PubMed]
- Walsh GL, Taylor GD, Nesbitt JC, et al. Intensive chemotherapy and radical resections for primary nonseminomatous mediastinal germ cell tumors. Ann Thorac Surg 2000;69:337-43; discussion 343-4. [Crossref] [PubMed]
- Kesler KA, Patel JB, Kruter LE, et al. The "growing teratoma syndrome" in primary mediastinal nonseminomatous germ cell tumors: criteria based on current practice. J Thorac Cardiovasc Surg 2012;144:438-43. [Crossref] [PubMed]
- Shintani Y, Kanzaki R, Kawamura T, et al. Surgical resection for advanced lung cancer using the hemi-clamshell approach. Interact Cardiovasc Thorac Surg 2017;25:462-8. [Crossref] [PubMed]
- Lardinois D, Sippel M, Gugger M, et al. Morbidity and validity of the hemiclamshell approach for thoracic surgery. Eur J Cardiothorac Surg 1999;16:194-9. [Crossref] [PubMed]
- Lebreton G, Baste JM, Thumerel M, et al. The hemiclamshell approach in thoracic surgery: indications and associated morbidity in 50 patients. Interact Cardiovasc Thorac Surg 2009;9:965-9. [Crossref] [PubMed]
- Fujiwara A, Funaki S, Ose N, et al. Surgical resection for advanced thymic malignancy with pulmonary hilar invasion using hemi-clamshell approach. J Thorac Dis 2018;10:6475-81. [Crossref] [PubMed]
- Masaoka A, Ito Y, Yasumitsu T. Anterior approach for tumor of the superior sulcus. J Thorac Cardiovasc Surg 1979;78:413-5. [Crossref] [PubMed]
- Erdös G, Tzanova I. Perioperative anaesthetic management of mediastinal mass in adults. Eur J Anaesthesiol 2009;26:627-32. [Crossref] [PubMed]
- Gothard JW. Anesthetic considerations for patients with anterior mediastinal masses. Anesthesiol Clin 2008;26:305-14. vi. [Crossref] [PubMed]
- Felten ML, Michel-Cherqui M, Puyo P, et al. Extracorporeal membrane oxygenation use for mediastinal tumor resection. Ann Thorac Surg 2010;89:1012. [Crossref] [PubMed]
- Inoue M, Minami M, Shiono H, et al. Efficient clinical application of percutaneous cardiopulmonary support for perioperative management of a huge anterior mediastinal tumor. J Thorac Cardiovasc Surg 2006;131:755-6. [Crossref] [PubMed]
- Yamashita S, Nakagawa M, Takaji K, et al. Giant yolk sac tumor operated under percutaneous cardiopulmonary support (PCPS) and aspiration device. Ann Thorac Cardiovasc Surg 2008;14:184-6. [PubMed]
Cite this article as: Sakakura N, Nakai A, Suda H, Nakada T, Matsui T, Nakanishi K, Shirai S, Nakada J, Horio Y, Oya Y, Takahashi Y, Kuroda H. Life-threatening massive bleeding in the pulmonary trunk adjacent to the right ventricular outflow tract during the resection of a large mediastinal germ cell tumor: proposed safety measures in the absence of cardiovascular surgeons: a case report. Mediastinum 2021;5:19.


