
A spectrum of Thymic mucosa-associated lymphoid tissue lymphoma and Thymic amyloidosis in the patient with Auto immune disease: a case series
Introduction
The thymus is an important organ for maintenance of systemic cellular immunity and prevention of autoimmune diseases (ADs) by production of mature T lymphocytes. It has a unique characteristic of spontaneous degeneration with age and displacement with fat tissue. The thymus has also been linked to disorders such as immunodeficiency; hematologic, endocrinologic, collagen, and neuromuscular defects; and AD, but the detailed mechanisms are unclear (1).
It is uncommon for tumors to originate from thymic tissue. The most common type is thymoma, with a 47% incidence of all mediastinum tumors in the anterior compartment in adults (1). In Japan, 2,365 cases of thymic tumors (thymoma, thymic cancer, thymic carcinoid and thymolipoma) were surgically treated in 2017, and 1,939 were cases of thymoma (82.0%) (2). Generally, thymic tumors are divided into four basic categories; epithelial cell, neuroendocrine cell, adipose tissue and miscellaneous tumors (1). On the other hand, several minor thymic tumors are also known, and in recent years we have surgically treated cases of thymic mucosa-associated lymphoid tissue (MALT) lymphomas (3) and localized thymic amyloidosis. All patients in these cases also had Sjögren’s syndrome (SjS), which suggests a possible link between these rare thymic tumors and SjS. Therefore, we reviewed cases with resected thymic tumors at our institute and affiliated hospital to examine the spectrum of these tumors in patients with ADs.
We present the following article in accordance with the AME Case Series Checklist (available at http://dx.doi.org/10.21037/med-20-68).
Methods
The clinical information of thymic amyloidosis and MALT lymphoma cases surgically treated at Kanagawa Cardiovascular and Respiratory Center, and Yokohama City University Hospital from January 2010 to December 2019 were reviewed. The correlation between resected thymic tumors at same period and coexistent ADs were also reviewed. The tumors were divided into four categories according to pathological findings: thymic epithelial tumors, lymphoproliferative lesions, mesenchymal tumors and non-neoplastic lesions. Non-neoplastic lesions (thymic hyperplasia, lymphoid hyperplasia and thymic cyst) that were suspected to be thymic tumors preoperatively were also defined as a thymic tumor. The clinical information and the follow-up were obtained on medical records and an outpatients’ clinic or phone call basis respectively. The inclusion criteria were all thymic tumors including non-neoplastic lesions diagnosed pathologically after surgery.
The correlation between each type of thymic tumor and AD was analyzed by Pearson χ2 test. Data are shown as a median and range. A P-value of <0.05 was considered to be significant. All analyses were performed using SPSS for Windows ver. 11.0 J (SPSS, Chicago, IL, USA).
This study is a retrospective and consecutive case series analysis of thymic tumor in multi center and was conducted in accordance with the Helsinki Declaration (as revised in 2013). This study was approved by the Kanagawa Cardiovascular and Respiratory Center Institutional Review Board for Clinical Research (approval number KCRC-20-0017). The informed consent requirement was waived because of the retrospective study design.
Results
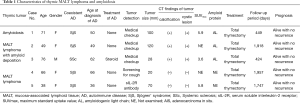
There were 5 cases of thymic amyloidosis and MALT lymphoma. The characteristics of the patients are shown in Table 1. The median age was 66 (range, 38–76) year-old, and 4 of the patients were female. Three cases (case 1, 3 and 5) had already diagnosed as ADs before detection of thymic tumors. Only SSc case was received preceding steroid medication. Two cases (case 2 and 4) diagnosed as SjS at the same time of the operation. Tumors were detected by medical checkup or by screening for cough and elevated serum soluble interleukin-2 receptor antibody.
Full table
The median maximum tumor diameter was 70 (range, 20–120) mm. Tumors which had contrasted effect contained solid part and some cystic parts at various rated on chest computed tomography (CT). Calcification was recognized with appearance of amyloid deposition. In (18F)-fluorodeoxyglucose positron emission tomography (FDG-PET) performed in 3 cases (cases 1, 3 and 5), the median maximum standard uptake value (SUVmax) was 5.8 (3.6–5.9). Four cases had hyperglobulinemia (cases 1, 2, 4 and 5) (data not shown). The amyloid protein was all AL type (cases 1, 2 and 3), which was compatible with localized amyloidosis (4,5). Two cases (cases 1 and 3) showed κ light chain dominant monoclonal overproduction of immunoglobulins (data not shown). In SjS cases, 3 of 4 had multiple lung cysts (cases 1, 2 and 4) on chest CT (data not shown).
In case 1, chest CT showed a solid, large, soft tissue density mass with calcification located in the anterior mediastinum (Figure 1A), and a grand glass opacity (GGO) lesion in the right upper lobe and multiple lung cysts in bilateral lung fields. FDG-PET showed increased uptake of FDG in the mediastinum tumor (SUVmax 5.9). Based on the radiological diagnosis of suspected thymoma and primary lung cancer, both of which were judged to be resectable, surgery via a median sternotomy was performed. The anterior mediastinum tumor was found to originate from the thymus gland and to be adhered to the pericardium firmly, but could be peeled off manually (Figure 1B). A GGO lesion in segment 3 of the right upper lobe was resected using staplers. An intraoperative histological examination of the thymic tumor revealed deposits of amorphous, eosinophilic materials, and no malignant cells. Finally, tumorectomy with thymectomy and right lung wedge resection were completed.
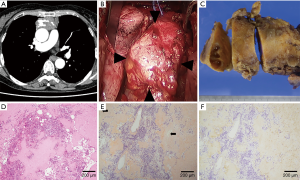
The resected thymic tumor measured 100 mm in maximum diameter, and had a smooth appearance and was firm. The cut surface was light-yellow with cystic lesions (Figure 1C). Histologically, small calcification foci and a small number of lymphocytes and plasma cells were observed in diffuse deposits of amorphous and eosinophilic material (Figure 1D). Hassall bodies were also seen, suggesting that the tumor developed in the thymus. The amorphous materials were positive in direct fast scarlet (DFS) 4BS staining and showed an “apple-green” birefringence under polarized light. These findings were compatible with amyloidosis (Figure 1E). Subsequent immunohistochemical staining showed that the amyloid material was positive for κ light chain (Figure 1F), slightly positive for transthyretin (TTR), and negative for λ light chain, amyloid A protein. Mass spectrography of amyloid material collected by laser microdissection confirmed that the amyloid consisted of immunoglobulin κ light chain (data not shown). In postoperative screening for systemic amyloidosis, an echocardiogram and examinations of gastric and colon fibers showed no abnormal findings. In urinalysis, Bence-Jones protein was not observed. Thus, diagnosis of localized amyloidosis arising from the thymus was confirmed. The GGO lesion was diagnosed as adenocarcinoma in situ.
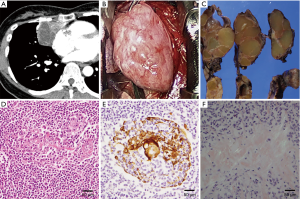
Cases 2 and 4 with thymic MALT lymphoma and SjS have been previously reported (3). In case 2, chest CT showed a large mass with a cystic lesion located on the right side of the heart toward the anterior mediastinum (Figure 2A) and multiple cysts in both lungs. In the operation, the tumor was found not to have invaded the surrounding structures and was easily removed (Figure 2B). Right lung wedge resection for sampling of the lung cystic lesions was also performed. The maximum diameter of the tumor was 120 mm, and it had a smooth surface. On sectioning, the tumor was solid yellowish, and was circumscribed by a thin fibrous capsule with small cysts containing clear yellowish fluid (Figure 2C). Histologically, the tumor consisted of diffuse monotonous proliferation of mononuclear cells and scattered atrophic lymphoid follicles. The mononuclear cells occasionally infiltrated into Hassall bodies to form lymphoepithelial lesions (LELs) (Figure 2D,E). Focal deposits of amorphous and eosinophilic materials were also scattered in the tumor. The deposits were positive on DFS 4BS staining and showed an “apple-green” birefringence under polarized light, suggesting amyloidosis (Figure 2F). These findings led to diagnosis of thymic MALT lymphoma with amyloid deposition. Lung cysts were bullae with amyloid deposits, consistent with lung manifestation of SjS (6,7).
Case 3 was the only male patient with MALT lymphoma and SSc. This case also had amyloid deposition. Coexistence of SSc and MALT lymphoma is extremely rare, with only 8 cases of SSc with MALT lymphoma at another site reported in the English-language literature (8). Cases 4 (3) and 5 had thymic MALT lymphoma with no amyloid deposition.
All patients were surgically treated with total thymectomy. The median postoperative follow-up period was 1,747 (range, 424–1,957) days and they are alive without recurrence, indicating a good prognosis.
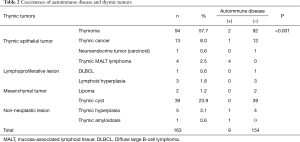
At the same period, there were 163 resected thymic tumor cases consisting of 94 thymomas (57.7%), 13 thymic cancers (8.0%), 1 neuroendocrine tumor (0.6%), 4 thymic MALT lymphomas (2.5%), 1 diffuse large B-cell lymphoma (0.6%), 3 lymphoid hyperplasias (1.8%), 2 lipomas (1.2%), 39 thymic cysts (23.9%), 5 thymic hyperplasias (3.1%) and 1 case of localized thymic amyloidosis (0.6%). In this series, 9 patients (5.5%) had ADs: 2 with thymoma (SjS), 1 with thymic cancer (SjS), 4 with thymic MALT lymphomas (3 SjS and 1 systemic sclerosis (SSc)), 1 with thymic hyperplasia (rheumatoid arthritis) and 1 with thymic amyloidosis (SjS) in our institute and the affiliated hospital at the same period. All 5 patients (3.1%) with thymic MALT lymphoma/amyloidosis had ADs (4 SjS and 1 SSc), and consequently there was a relation between ADs and thymic MALT lymphoma/amyloidosis (P<0.001) (Table 2).
Full table
Discussion
Thymic amyloidosis defined as “primary localized amyloidosis arising from the thymus, not a part of manifestation of systemic amyloidosis” is extremely rare (1), with only six cases reported in the English-language literature (9-14): a case of sclerosing thymoma-like amyloidoma (9), two cases combined with myasthenia gravis (10,11), and a case of thymic amyloidosis with diplopia that resolved immediately after thymectomy (12). These cases included 3 AL (amyloidgenic light chain) types, 1 AA (amyloidogenic A protein) type with rheumatoid arthritis, and 2 cases that were negative for AL and AA amyloidosis. Chest CT showed a soft tissue density mass with calcification in all 6 previous cases, and in our case (case 1). Complete resection is thought to be the best treatment for a localized lesion, with five of the previous cases of thymic amyloidosis undergoing thymectomy. The surgical procedure was not indicated in the case of sclerosing thymoma-like thymic amyloidoma (9) due to advanced thymoma (Masaoka stage IVa pericardial dissemination) and the patient was treated with steroid therapy only. Although “calcification in 20% to 50% of lung nodular amyloidosis” (15) and “increased uptake of FDG in 70% of other-site AL amyloidosis with a median SUVmax of 6.5 and no uptake in patients with cardiac amyloidosis and pulmonary amyloidosis” (16) were previously reported, the detailed etiology and clinical manifestations of thymic amyloidosis are still unknown.
Thymic MALT lymphoma is a type of extranodal marginal zone B cell lymphoma (MZBL) defined by Isaacson in 1990 (17). Unlike at other sites, thymic MALT lymphoma has unique characteristics, including geographic and gender concentration in Asian women, an association with ADs (>60% of cases, especially SjS), and monoclonal gammopathy of IgA (3,18-20). Radiologically, the tumor appears as a solid mass with cystic changes in the anterior mediastinum on chest CT and magnetic resonance imaging (MRI) (18). Previous studies have reported a median SUVmax of 6.0 (range, 0.7–28.0) for MALT lymphoma, but with PET activity depending on the organ site (i.e., thyroid lesions, high; skin lesions, low) (21); however, no data for thymic MALT lymphoma are available.
Pathologically, extensive infiltration of lymphocytes with a small fraction of centrocyte-like (CCL) cells to Hassall bodies (i.e., LELs) are a distinctive finding. The reasons for the clinical bias and etiology are unknown, but several theories for the etiology have been proposed. For example, an AD, such as SjS, promotes continuous replication of B lymphocytes that can lead to development of nodular lymphoid hyperplasia and subsequent neoplastic transformation in the thymic gland (8,18,20). Ströbel et al. (22) also speculated that abnormal production of chemoattractants by the thymic epithelia might contribute to MALT lymphoma development. Thymic MALT lymphoma has as excellent prognosis with a 5-year survival rate of 83%, indicating that surgical resection and/or chemotherapy is effective (1,19,20). Furthermore spontaneous regression may occur based on a watch and wait follow-up study, although we note that this was for a case of lung MALT lymphoma (23).
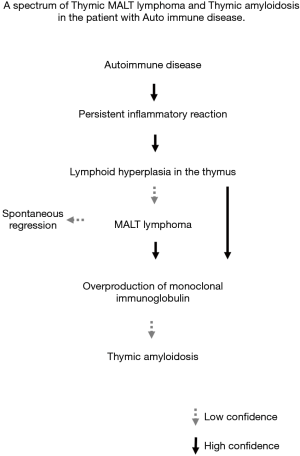
Although the causal relationship has not been determined, several studies have shown an association of AD with pulmonary MALT lymphoma and pulmonary amyloidosis (22,24-26). To our knowledge, no study has associated with the relationship of these rare thymic tumors with AD. In our investigation, there was a relation between ADs and thymic MALT lymphoma/amyloidosis (P<0.001). Based on our experience, we propose a process of tumorigenesis from thymic MALT lymphoma to amyloidosis (Figure 3). Underlying AD causes a persistent or chronic inflammatory reaction, and lymphoid hyperplasia may then develop in the thymus. Some cases develop MALT lymphoma (cases 4, 5) and this causes overproduction of immunoglobulins in the form of κ or λ light chains that are misfolded and deposited in thymic tissue. In this phase, a pathological examination shows both MALT lymphoma and amyloid deposition (cases 2, 3). MALT lymphoma can show spontaneous regression (23) and may disappear in some cases, leaving only amyloid tissue to be seen pathologically in this phase (case 1). Calcification was seen dominantly in amyloid deposition cases on CT. It is possible that Calcium deposition might be caused by MALT lymphoma degeneration. All MALT lymphomas were easily removed whereas amyloidosis was fixed firmly to the pericardium. This intraoperative findings suggested that MALT lymphoma regression might case fibrosis leading to adhesion to the surrounding structures in the sequential phase. In some thymic amyloidosis cases, combined resection with surrounding organs (i.e., lungs, vessels and pericardium) might be necessary.
Conclusions
When the clinician encounters a patient with AD, especially SjS including subclinical case, routine chest CT is recommended and may provide thymic tumors. Conversely, in case of mediastinum tumor, screening test for AD is also recommended. In conclusion, our study shows a possible spectrum of thymic MALT lymphoma and thymic amyloidosis, in which ADs, especially SjS, might be important underlying conditions in tumorigenesis of these rare tumors. A further study is warranted to examine this hypothesis.
Limitation
The major limitation of this study is the small series.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the AME Case Series Checklist. Available at http://dx.doi.org/10.21037/med-20-68
Peer Review File: Available at http://dx.doi.org/10.21037/med-20-68
Conflict of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-68). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study is a retrospective and consecutive case series analysis of thymic tumor in multi center and was conducted in accordance with the Helsinki Declaration (as revised in 2013). This study was approved by the Kanagawa Cardiovascular and Respiratory Center Institutional Review Board for Clinical Research (approval number KCRC-20-0017). The informed consent requirement was waived because of the retrospective study design.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shields TW, LoCicero J, Reed CE, et al. General Thoracic Surgery, 7th ed. Philadeiphia: Lippincott Williams & Wilkins, 2009.
- Committee for Scientific Affairs. Thoracic and cardiovascular surgeries in Japan during 2017: Annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2020;68:414-49. [Crossref] [PubMed]
- Arai H, Tajiri M, Kaneko S, et al. Two surgical cases of thymic MALT lymphoma associated with multiple lung cysts: possible association with Sjögren’s syndrome. Gen Thorac Cardiovasc Surg 2017;65:229-34. [Crossref] [PubMed]
- Pepys MB. Amyloidosis. Annu Rev Med 2006;57:223-41. [Crossref] [PubMed]
- Grogg KL, Aubry MC, Varana JA, et al. Nodular Pulmonary Amyloidosis Is Characterized by Localized Immunoglobulin Deposition and Is Frequently Associated With an Indolent B-cell Lymphoproliferative Disorder. Am J Surg Pathol 2013;37:406-12. [Crossref] [PubMed]
- Takahashi K, Sadamatsu H, Ogusu S, et al. Sjögren Syndrome Complicated with Cystic Lung Disease and Pulmonary Amyloidosis. Case Rep Rheumatol 2018;2018:7475242 [Crossref] [PubMed]
- Gómez Correa GA, Osorno Serna J, Cáceres Acosta MF, et al. Nodular Pulmonary Amyloidosis: A Manifestation of Sjögren's Syndrome. Case Rep Pulmonol 2018;2018:9745935 [Crossref] [PubMed]
- Fukumoto T, Matsuoka H, Kitani M, et al. Coexistence of mucosa-associated lymphoid tissue lymphoma and systemic sclerosis showing positive for anticentromere antibody and anti-RNA polymerase III antibody: A case report and published work review. J Dermatol 2018;45:e337-9. [Crossref] [PubMed]
- Kato Y, Okuda M, Fukuda K, et al. Sclerosing thymoma-like thymic amyloidoma with nephrotic syndrome: a case report. J Med Case Rep 2017;11:216. [Crossref] [PubMed]
- Chapman KO, Beneck DM, Dinkin MJ. Ocular Myasthenia Gravis With Thymic Amyloidosis. J Neuroophthalmol 2016;36:50-2. [Crossref] [PubMed]
- Son SM, Lee YM, Kim SW, et al. Localized Thymic Amyloidosis Presenting with Myasthenia Gravis: Case Report. J Korean Med Sci 2014;29:145-8. [Crossref] [PubMed]
- Sato F, Hata Y, Otsuka H, et al. Isolated Nodular Thymic Amyloidosis Associated With Diplopia. Ann Thorac Surg 2014;98:1470-2. [Crossref] [PubMed]
- Ha SY, Lee JJ, Park HJ, et al. Localized Primary Amyloidosis Presenting as a Mediastinal Mass: A Case Report. Korean J Pathol 2011;45:S41-4. [Crossref]
- Takamori S, Yano H, Hayashi A, et al. Amyloid Tumor in the Anterior Mediastinum: Report of a Case. Surg Today 2004;34:518-20. [Crossref] [PubMed]
- Müller NL, Silva CIS. Imaging of the chest. Philadeiphia: Saunders Elsevier, 2009.
- Mekinian A, Jaccard A, Soussan M, et al. 18F-FDG PET/CT in Patients With Amyloid Light-Chain Amyloidosis: Case-Series and Literature Review. Amyloid 2012;19:94-8. [Crossref] [PubMed]
- Isaacson PG, Chan JK, Tang C, et al. Low-grade B-cell lymphoma of mucosa-associated lymphoid tissue arising in the thymus. A thymic lymphoma mimicking myoepithelial sialadenitis. Am J Surg Pathol 1990;14:342-51. [Crossref] [PubMed]
- Shimizu K, Yoshida J, Kakegawa S, et al. Primary Thymic Mucosa-Associated Lymphoid Tissue Lymphoma Diagnostic Tips. J Thorac Oncol 2010;5:117-21. [Crossref] [PubMed]
- Inagaki H, Harris NL, Chan JKC, et al. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). In: Travis WD, Brambilla E, Burke AP, et al. editors. WHO Classification of Tumors of the Lung, Pleura, Thymus and Heart. 4th ed. Lyon: International Agency for Research on Cancer (IARC), 2015:270-1.
- Inagaki H, Chan JK, Ng JW, et al. Primary thymic extranodal marginal-zone B-cell lymphoma of mucosa-associated lymphoid tissue type exhibits distinctive clinicopathological and molecular features. Am J Pathol 2002;160:1435-43. [Crossref] [PubMed]
- Qi S, Huang MY, Yang Y, et al. Uptake of [18F]fluorodeoxyglucose in initial positron-emission tomography predicts survival in MALT lymphoma. Blood Adv 2018;2:649-55. [Crossref] [PubMed]
- Ströbel P, Marino M, Feuchtenberger M, et al. Micronodular Thymoma: An Epithelial Tumour With Abnormal Chemokine Expression Setting the Stage for Lymphoma Development. J Pathol 2005;207:72-82. [Crossref] [PubMed]
- Troch M, Streubel B, Petkov V, et al. Dose MALT Lymphoma of Lung Requier Immediate Treatment? An Analysis of 11 Untreated Cases with Long-term Follow-up. Anticancer Res 2007;27:3633-7. [PubMed]
- Corrin B, Andrew GN. Pathology of the Lungs. 3rd ed. Lodon: Elsevier, 2011.
- Lim JK, Lacy MQ, Kurtin PJ, et al. Pulmonary marginal zone lymphoma of MALT type as a cause of localized pulmonary amyloidosis. J Clin Pathol 2001;54:642-6. [Crossref] [PubMed]
- Tschang TP. Nodular Malignant Lymphoma and Amyloidosis. A Case Report. Cancer 1976;38:2192-6. [Crossref] [PubMed]
Cite this article as: Arai H, Tajiri M, Kikunishi N, Nakamura S, Inafuku K, Ishikawa Y, Ikeda S, Sekine A, Okudela K, Iwasawa T, Masuda M. A spectrum of Thymic mucosa-associated lymphoid tissue lymphoma and Thymic amyloidosis in the patient with Auto immune disease: a case series. Mediastinum 2021;5:12.


