
Five years of thoracic endoscopy unit activity on lung cancer staging: how teamwork can improve the outcomes
Introduction
Lung cancer is the leading cause of cancer related deaths in the world. Non-small cell lung cancer (NSCLC) represents 80% of all lung cancers. Despite all recent therapeutic advances, patients with NSCLC still present an estimated 5-year overall survival rate in less than 25% of cases for all stages. Current prognostic factors for this tumor include the TNM staging, tumor grade, tumor size, histology subtype which need to be correlated to other biomarkers.
An accurate lung cancer patient staging is crucial to conduct an appropriate treatment. An accurate staging is based first of all, on a total body CT scans and a FDG-PET to establish the absence of multiple pulmonary lesions and the absence of hepatic, adrenal, or brain metastases and to evaluate hilar and mediastinal lymph node status (1).
Endobronchial Ultrasound Transbronchial Needle Aspirations (EBUS-TBNA) have proven to be highly sensitive, specific and safe in the staging of lung cancer patients (2).
Endoscopic Ultrasound Fine Needle Aspirations (EUS-FNA) plays an important role in the diagnosis and staging of thoracic diseases, including lung cancer. It is considered the most effective technology to visualize and to obtain samples from suspected mediastinal lymph nodes metastases in some mediastinal stations (3).
In this study we analysed all patients who underwent mediastinal lymph nodes biopsies by endoscopic procedures in our thoracic endoscopy unit while paying attention to the learning curves of these five years of activity. We present the following article in accordance with the MDAR checklist (available at http://dx.doi.org/10.21037/med-20-53).
Methods
We retrospectively reviewed the diagnostic performance of EUS-FNA and EBUS-TBNA biopsies in a cohort of patients referred to our oncological centre between January 2013 and February 2018. This study was submitted and approved by the Internal Review Board of our Institution (nr. 1338/20) and was conducted in agreement with the Declaration of Helsinki. Informed consent was waived because of the retrospective nature of this study.
Statistical analysis
Data analysis was done with SPSS software (Version 23.0), mean plus standard deviation were calculated for numerical variables, and the frequency and percentage were reported for categorical factors. The sensitivity, specificity, and accuracy were calculated associated to positive predictive value (PPV) and negative predictive value (NVP). We then analysed the learning curve using accuracy as standard value.
Pre-workup evaluation
We evaluated all patients with suspicious mediastinal malignancies that were discussed in our thoracic endoscopy unit composed by thoracic surgeons, thoracic endoscopists, dedicated radiologists, and pathologists. We usually investigated N2 stations and N1 stations and we sampled all sonically evident mediastinal lymph nodes. We used EUS-FNA in case of enlarged mediastinal lymph nodes in stations 4L,5,7,8,9, and 3P. In the other stations as paratracheal stations [2, 4], hilum stations [10, 11] EBUS-TBNA is indicated.
According to the oncological lung guidelines, potentially pathological (defined abnormal) lymph nodes observed at CT scan are those with a short axis diameter over 1 cm and/or positive FDG-PET activity with standard uptake value of 2 or greater (4). Prior diagnostic evaluation included conventional work-up with medical history, physical examination, laboratory tests, electrocardiogram and eventually bronchoscopy (5). Based on the latest CT scan we decided which procedure to perform. In case of enlarged lymph nodes in station 7 which were reachable with both techniques we preferred to use EUS-FNA for lesser invasiveness and better tolerability (6).
All procedures were done by well-trained operators in an outpatient setting with an observation time after the procedures 6 hours approximately. Patients signed an informed consent before the procedure.
EUS-FNA technique
Endoscopic ultrasound was performed with a dedicated linear echo-endoscope probe connected to the ultrasound unit. The echo-endoscope is initially introduced up to the level of the coeliac axis and gradually withdrawn upwards for a detailed mediastinal imaging. A systematic examination of every mediastinal lymph node station was achieved (7).
EBUS-TBNA technique
The EBUS-TBNA procedure was performed by an EBUS-guided TBNA bronchoscope by the oral route under sedation with intravenous infusion of propofol with accurate monitoring of patient parameters. Local oropharynx anaesthesia was obtained by using 1% lidocaine spray and gel. Mediastinal and hilar lymph nodes were examined systematically. At least three consecutive aspirates were obtained from each lymph node station (8).
Pulse and colour Doppler ultrasonography imaging were used to avoid vascular structures during insertion of the needle. We routinely used a 22-gauge needle; sometimes we utilized a 19-gauge needle or 22-gauge procore needle (Cook echo tip). The aspiration technique was used in all cases. After introducing the needle into the lesion, the stylet was gently removed from the needle and a syringe was attached allowing negative pressure to aspirate cells; the needle was moved within the target lesion back and forth for about 15 to 20 times.
Results
Between January 2013 and February 2018, we performed a total of 929 endoscopic procedures, 432 EBUS-TBNA and 497 EUS-FNA. Biopsy was performed at the following mediastinal sites according to the regional lymph-node map definitions: biopsies at station 2 were performed in 89 patients; at station 3P in 27 cases, at station 4 in 571 patients (457 4R and 114 4L); we performed biopsy at station 5 in 52 cases; at station 7 in 642 cases, at stations 8 and 9 in 211 cases. Hilar stations, 10 and 11, were reachable with EBUS and biopsies were performed in 547 cases, 389 on station 10 and 158 on station 11.
The final diagnosis included: NSCLC (n=645, 76.69%), small cell lung cancer (SCLC) (n=190, 22.59%), neuroendocrine tumor (n=5, 0.60%) and mesothelioma (n=1, 0.12%). In 88 cases, we had negative diagnoses (no tumor cells); in 52 cases specimens were adequate; in 36 cases, specimens were considered inadequate (amorphous material and fibrosis) and surgery (VATS and Chamberlain mediastinotomy) was necessary to obtain final diagnosis of NSCLC in 12 cases, SCLC in 14 cases and no tumor cells in 10 cases.
EUS-FNA biopsies had a sensitivity, specificity, accuracy, PPV and NVP of 95.5%, 97%, 93.5%, 98.7% and 66.2% respectively. EBUS-TBNA biopsies had a sensitivity, specificity, accuracy, PPV and NVP of 92.5%, 96.3%, 91%, 98.1% and 68%. The association of EBUS-TBNAb and EUS-FNAb presented a sensitivity, specificity, accuracy, PPV and NVP of 97.0%, 100%, 96%, 70.4% and 100% respectively (Table 1). The diagnostic accuracy of EBUS-TBNAb combined with EUS-FNAb was significantly higher than that of a single diagnostic method (P<0.05). Then, we evaluated the accuracy of both the techniques in each mediastinal station. The results, summarised in the Table 2, showed a higher accuracy in the station 7 compared to the other stations as it was reachable with both techniques. We evaluated the accuracy of EBUS-TBNAb and EUS-FNAb on station 7 biopsies and results showed that EUS-FNAb had a higher accuracy but not significantly compared to EBUS-TBNAb technique (Table 3). We also evaluated the diagnostic yield of both techniques on station 4L. In this case, EBUS-TBNAb showed a higher accuracy, but not significant compared to EUS-FNAb (Table 3). Regarding to hilar stations, reachable only with EBUS, we found an accuracy of 92.5% on station 10 and 91% on station 11.
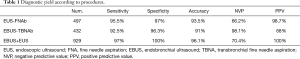
Full table
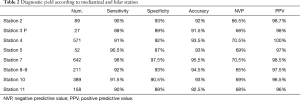
Full table

Full table
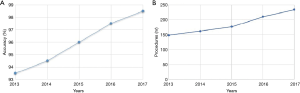
Then, we analysed the accuracy of both procedures during the first five years of activity. Results are summarised in the Figure 1. The curves trend showed a constant improvement, both in terms of numbers of procedure and global accuracy, especially in the two-years period 2016–2017. Therefore, we selected the patients who have been followed at our institution since the first visit to the definitive treatment (nr.=798) end, we evaluated the time from first outpatient clinic visit to the final treatment (surgical treatment or medical treatment). We noticed that in the biennium 2016–2017, time was significantly lower compared to the years 2013–2015 (24.5±6 vs. 29.5±7 days; P<0.05)
Discussion
Before the advent of EBUS-TBNA and EUS-FNA, mediastinoscopy had been the mainstay for investigating abnormal mediastinal lymph nodes (9). With the introduction of endoscopy techniques, mediastinoscopy was relegated as second level procedure, in case of undetermined results with endoscopy. As well as ESTS guidelines recommend regarding the best clinical practice for the lung cancer patient staging, the association between EBUS and EUS allows to improve the quality standard of diagnosis in terms of sensitivity, specificity and accuracy. From the first introduction of these techniques in the diagnosis of lung cancer, we decided to institute a thoracic endoscopy unit composed by thoracic surgeons, digestive endoscopists and dedicated pathologists (10). This enabled to accelerate the workup process from the first outpatient visit to the final treatment through an accurate diagnosis and a marked improvement of our quality standards in the process of lung cancer patient staging. During the first five years of experience of our thoracic endoscopy unit, we performed a high volume of procedures and we were able to decrease the number of surgeries for diagnosis and staging. Therefore, we were able to dedicate more operating space to curative surgeries for lung cancer, reducing the waiting time. With these results we confirmed that an oncological thoracic endoscopy unit is essential to increase the quality of care for patients with lung cancer.
Regarding the diagnostic yield of the endoscopic procedures on single stations, we highlighted that subcarinal station is more easily reachable with EUS. Indeed, the oesophageal wall allows multiple movements to the instrument compared to the less mobile structure of the trachea and this could make easier the sampling of lymph nodes tissue. On the station 4L, reachable by both techniques, accuracy is non-significantly higher for EBUS, for the extreme proximity of lymph nodes to the trachea.
Paramount focus should be done concerning the analyse of the learning curves. The constant increase of procedures over the five years has led to an improvement in the global accuracy of the diagnosis. Every practitioner that composed our team, has contributed equally to achieving these results. From 2013, procedures were performed by a thoracic surgeon and a digestive endoscopist and combining their skills made it possible to quickly learn techniques with a high-quality diagnostic yield. As our curves demonstrated, the thoracic surgeon skills in terms of knowledge of anatomical structures of the mediastinum combined to the digestive endoscopist skills in terms of the eco-endoscopic instruments management, has led us to raise the quality standards of the care of lung cancer patients. Two dedicated nurses are part of our unit, premising a quick preparation of the patient and an adequate support for the operator. A dedicated pathologist standardised the techniques for the tissue sampling. We did not use an on-site cytologist and the adequate sampling was insured performing three to six needle passes for each lesion. The first evaluation of the tissue sampling adequacy was evidenced by the presence of a long strain of tissue (>1 cm) in the test tube, without the presence of large amount of blood. All patients were selected by our dedicated anesthesiologist that evaluated the specific risks of each patient to undergo the procedures. That allowed us to minimize the risk of post procedures complications. After the procedures, patients were monitored three to six hours evaluating O2 saturation, blood pressure and heart rate.
Last but not the least, is the evaluation of time from diagnosis to treatment. We selected only patients that had been followed at our institution for the entire medical care. The significantly lower time of biennium 2016–2017 confirmed that a well-functioning and well-trained thoracic endoscopy unit can increase the quality standards of cancer care. We highlight that the thoracic surgeon’s role starts from outpatient clinic until the final treatment, passing from diagnostic procedures, placing his expertise more and more as a reference point for the patient with lung cancer.
In conclusion, the institution of a thoracic endoscopy unit with the possibility to perform both procedures by digestive endoscopists and thoracic surgeons, allowed to increase the number of endoscopic procedures and improve the sensitivity and the accuracy of the minimally invasive hilar-mediastinal staging. The practitioners that composed the team should always be the same, as the experience gained increases the diagnostic accuracy of the procedures.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR checklist. Available at http://dx.doi.org/10.21037/med-20-53
Data Sharing Statement: Available at http://dx.doi.org/10.21037/med-20-53
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-53). FF serves as an unpaid editorial board member for this journal from Apr 2020 to Mar 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was submitted and approved by the Internal Review Board of our Institution (nr. 1338/20) and was conducted in agreement with the Declaration of Helsinki. Informed consent was waived because of the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee JW, Kim EY, Kim DJ, et al. The diagnostic ability of 18F-FDG PET/CT for mediastinal lymph node staging using 18F-FDG uptake and volumetric CT histogram analysis in non-small cell lung cancer. Eur Radiol 2016;26:4515-23. [Crossref] [PubMed]
- Sakairi Y, Nakajima T, Yoshino I. Role of endobronchial ultrasound-guided transbronchial needle aspiration in lung cancer management. Expert Rev Respir Med 2019;13:863-70. [Crossref] [PubMed]
- Annema JT, Versteegh MI, Veseliç M, et al. Endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of lung cancer and its impact on surgical staging. J Clin Oncol 2005;23:8357-61. [Crossref] [PubMed]
- Labarca G, Aravena C, Ortega F, et al. Minimally Invasive Methods for Staging in Lung Cancer: Systematic Review and Meta-Analysis. Pulm Med 2016;2016:1024709 [Crossref] [PubMed]
- Call S, Obiols C, Rami-Porta R. Present indications of surgical exploration of the mediastinum. J Thorac Dis 2018;10:S2601-10. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Preoperative mediastinal lymph node staging for non-small cell lung cancer: 2014 update of the 2007 ESTS guidelines. Transl Lung Cancer Res 2014;3:225-33. [PubMed]
- Witte B, Neumeister W, Huertgen M. Does endoesophageal ultrasound-guided fine-needle aspiration replace mediastinoscopy in mediastinal staging of thoracic malignancies? Eur J Cardiothorac Surg 2008;33:1124-8. [Crossref] [PubMed]
- Coutinho D, Oliveira A, Campainha S, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for nodal staging in non-small cell lung carcinoma. Rev Port Pneumol 2006;2017:85-9. [PubMed]
- Hujala KT, Sipilä JI, Grénman R. Mediastinoscopy--its role and value today in the differential diagnosis of mediastinal pathology. Acta Oncol 2001;40:79-82. [Crossref] [PubMed]
- Jacobsen MM, Silverstein SC, Quinn M, et al. Timeliness of access to lung cancer diagnosis and treatment: A scoping literature review. Lung Cancer 2017;112:156-64. [Crossref] [PubMed]
Cite this article as: Gallina FT, Assisi D, Forcella D, Pierconti F, Visca P, Melis E, Facciolo F. Five years of thoracic endoscopy unit activity on lung cancer staging: how teamwork can improve the outcomes. Mediastinum 2021;5:13.


