
A thymoma or not a thymoma—that is the question: a case report
Introduction
Thymomas are rare, epithelial tumors of the thymus of diverse morphology. The WHO histological classification includes five main subtypes A, AB, B1, B2 and B3 and some less common types e.g., micronodular thymoma with lymphoid stroma and metaplastic thymoma. Types A and AB usually harbour a specific GTF2I gene mutation. In one third of thymoma cases autoimmune disorders are diagnosed, mainly myasthenia gravis (1).
All but the micronodular subtype are clearly malignant tumors since they may metastasize, relapse after resection or cause the death of a patient in progressing cases. Resection is the treatment of choice but advanced tumors require neoadjuvant approaches or adjuvant radiotherapy after surgery or even chemotherapy.
Thymolipomas are benign tumors composed of thymic parenchyma and adipose tissue. Some tumors may contain muscle or may show foci of sebaceous differentiation. The tumors are encapsulated and well demarcated, and often they are very large. These tumors are extremely rare and usually reported in the literature as case reports. Genetic abnormalities are not known, however, in one case a translocation of the HMGA2 gene was found. Some thymolipomas are accompanied by myasthenia gravis or other autoimmune diseases (1-5). Thymolipomas are treated by resection and they do not require subsequent additional treatment. Prognosis is excellent because the tumors do not progress.
The differentiation between thymoma and thymolipoma is crucial due to their distinct clinical course and therapeutic approach.
We present the case of a mediastinal tumor that shares the features of a thymoma of unknown histological type and a thymolipoma-like tumor. We explore arguments for and against both types of tumors, but the final diagnosis was not established, even after an extensive discussion among several expert pathologists during the 2019 International Thymic Malignancy Interest Group (ITMIG) Annual Meeting. We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/med-2021-01).
Case presentation
A 37-year-old male with metabolic syndrome and allergy especially to animal hair underwent a computed tomography angiography (CT angiography) after a severe attack of breathlessness to differentiate between an asthmatic episode and a pulmonary artery embolism.
CT analysis excluded pulmonary vessel pathology but revealed a slightly heterogeneous, solid mass containing some low-density fatty bands within the thymus. Neither calcifications nor features of vascular infiltration were detected. The thymus in the vicinity of the tumor was slightly enlarged with rounded edges. The preferred diagnosis among the differential diagnostic options for a solid, fat-containing mass in the anterior mediastinum was a teratoma (Figure 1).
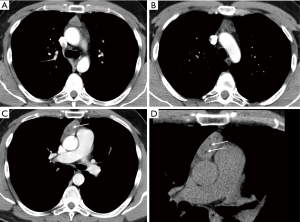
A thymectomy was performed. The resected tumor measured 5.5×4.5×2 cm and was well-delineated without macroscopic evidence of an infiltration into the adjacent fatty tissue.
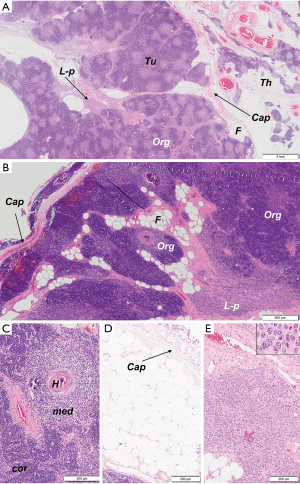
Microscopic analysis revealed an encapsulated tumor of heterogenic but ordered appearance. Three components of the tumor were identified: (I) a highly organoid component that reproduced the thymic parenchyma with medullary and cortical differentiation and numerous Hassall corpuscles but with an increased ratio of medullary to cortical areas (about 75% of the tumor surface); (II) a lymphocyte-poor, epithelial component composed of sheets of densely packed, elongated cells with inconsiderable nuclear atypia (about 10%); (III) mature adipose tissue (about 15%) (Figure 2).
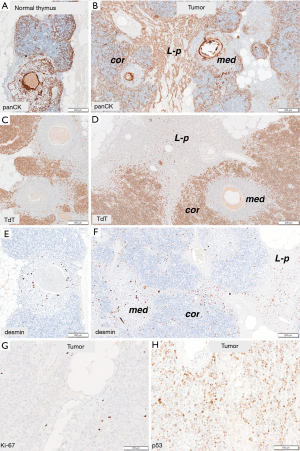
Immunohistochemical tests with cytokeratins (pancytokeratin, CK7, CK19 and CK5/6) and squamous differentiation marker (p40) showed in thymic-like component a slightly denser reaction in comparison to normal thymic parenchyma but of similar distribution. Anti-Beta5t and anti-AIRE reactions revealed normal number of cortical and medullary cells, respectively. Like in a normal thymus, numerous myoid cells were detected in medullary areas in an anti-desmin reaction. They were also present in lymphocytes-poor areas. CD20 was negative in epithelial cells, but highlighted small agglomerates of B-lymphocytes in medullary areas of both tumor and thymic tissue. The epithelial cells of at the border of cortical areas in thymic-like component and of lymphocyte-poor component were D2-40-positive. Ki-67 and p53 indexes could be assessed only in lymphocyte-poor component and reached 1% and 30%, respectively (Figure 3).
The adipose tissue that was found inside the tumor, was admixed with other tumor components. The whole tumor was subtotally surrounded by a fibrous capsule. In some areas the capsule was missing, but still a tumor margin could be easily identified.
Genetic analysis of GTF2I, BRAF and NRAS genes was performed and revealed no mutations.
A definite diagnosis was not established, because the tumor did not meet the microscopic criteria of any of the established thymic tumor entities. Instead, the final histopathological report included two of the most probable diagnoses: a highly organoid thymoma, not otherwise specified (NOS) and a thymolipoma-like tumor. The first option implied that the fatty tissue should not be regarded as a component of the tumor but that there was an invasion into the mediastinal fat, i.e., stage pT1a (TNM, 8th Ed.)/IIB (Masaoka-Koga staging system) disease. By contrast, the second option implied that the fatty tissue constituted a component of the tumor without signs of an invasion.
The resection was complete.
Taking the highly organoid structure of the tumor, and its complete resection into account, the oncologists refrained from adjuvant radiotherapy. Control magnetic resonance imaging (MRI) studies were performed every three months. At the moment of this publication (a year and half after surgery) there is no evidence of tumor recurrence.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images.
Discussion
We present the case of a unique thymic tumor of unknown malignant potential, not included in the recent WHO histological classification (2015) (1). We also could not find a similar case described in the literature.
The tumor shared the features of invasive thymoma and thymolipoma-like tumor and was clearly different from the major lymphocyte-rich thymoma types, i.e., type AB, B1 and B2 thymoma, while type A and B3 thymomas could be excluded due to their lymphocytes-poor nature. Micronodular thymoma with lymphoid stroma and metaplastic thymoma are tumors of well-defined, very specific morphology completely different from the tumor that we present here.
The most organoid subtype of a thymoma that largely reproduces the normal juvenile thymus is type B1 thymoma. It contains medullary and cortical areas with a predominance of regions with cortical differentiation. Like the cortex of the normal thymus, cortical areas of type B1 thymomas contain abundant, densely packed TdT-positive (immature) T cells and a minority of interspersed epithelial cells that express Beta5t. The medullary areas (islands) are characterized by mature, TdT-negative T lymphocytes, aggregates of B lymphocytes and variable numbers of desmin-positive myoid cells. In a subset of type B1 thymomas a minority of epithelial cells in medullary islands express AIRE (autoimmune regulator)—a marker of advanced medullary differentiation (1,6). The main component of the tumor in our case, similarly to type B1 thymoma, showed organoid features with medullary and cortical differentiation; however, medullary areas predominated. Moreover, our tumor concomitantly contained almost pure epithelial, lymphocyte-poor areas that are not found in type B1 thymomas. The cells of this component expressed neither AIRE nor Beta5t. Instead, these sheets of epithelial cells showed D2-40-positivity that is a feature described in B2 thymomas and cortical areas of the thymus (7).
The current tumor was also clearly different from type AB thymoma that is composed of both lymphocyte-rich and lymphocyte-poor components in different proportions. The lymphocyte-rich component may rarely contain inconspicuous medullary islands, which usually do not contain CD20-positive B lymphocytes and lack myoid cells. Hassall corpuscles are normally absent. In our case, medullary areas showed features consistent with normal thymus and Hassall corpuscles were numerous. The epithelial cells of type AB thymomas often express CD20 in immunohistochemical tests, and genetic analysis reveals the unique p.L424H mutation of the GTF2I gene in over 70% of cases (8). In the current tumor we did not find CD20 expression and a GTF2I mutation.
One of the most intriguing aspects of the current tumor was its association with mature adipose tissue that was found inside the tumor around and in between thymus-like lobules. This is a common finding in advanced thymomas when they penetrate the capsule and infiltrate the adjacent tissues. In our case however, fatty tissue was found within the encapsulated tumor in both radiological and microscopic examination. Tumors that are composed of thymic parenchyma (non-neoplastic) and a fatty tissue regardless the proportions of both components are named thymolipomas (2,9). Radiologically, tumors of anterior mediastinum containing fat are either thymolipomas or teratomas. Microscopically, the features of the current tumor were not compatible with teratoma, since it was exclusively composed of adipose tissue and elements of the thymic gland, although abnormal in morphology.
The etiology of thymolipomas is unknown. Some authors suggest an origin from thymic adipose tissue or thymic true hyperplasia (10,11). In one case a translocation in HMGA2 gene was encountered, i.e., a genetic aberration that is often found in lipomas (5). Thymolipomas may contain components other than thymus—tumors harbouring skeletal muscle, myoid cells or foci of sebaceous differentiation have been reported (3,4,12). In one case, named thymohemangiolipoma, multiple medium-caliber blood vessels were found (13). Thymofibrolipoma is a histological variant of thymolipoma with abundant fibrous tissue (14,15). Rarely, conventional thymomas have been observed inside thymolipomas (16,17).
The thymic component in thymolipomas is by definition non-neoplastic, although molecular evidence for this assumption is largely lacking (1). In our case slight nuclear atypia of epithelial cells in lymphocyte-poor component and the marked discrepancy between very low Ki67 index and elevated p53 index favour the diagnosis of neoplasm, but it remains unclear whether it is a benign or malignant process. Therefore, we use the descriptive term thymolipoma-like only to highlight the morphological resemblance to thymolipoma.
The highly organoid structure and minimal atypia of the current tumor suggest a low malignant potential or maybe even a benign nature of the tumor. Taking this interpretation and the completeness of a resection into account, no adjuvant radiotherapy was recommended and the patient has been controlled radiologically every three months since surgery. At the time of this publication, one and half year after surgery, there were no signs of recurrence. Nevertheless, a long-term follow-up appears justified, considering the fact that thymomas may recur or metastasize even 10–15 years after resection of the primary tumor.
In summary, we presented the clinical, radiological and microscopic features of a unique thymic tumor not described in recent WHO histological classification of thymic tumors (1). We also did not find similar case in the literature. Despite careful immunohistochemical and basic genetic examination it was impossible to establish unequivocally the biological character of the tumor and to make a clear distinction between an unusual thymoma and thymolipoma-like lesion. The clinical outcome will be decisive. We present this case to make other pathologists pay special attention to similar lesions. The collection of further such cases could provide deeper insights into their biology and enable optimal therapy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mirella Marino, Brett W. Carter) for the series “Dedicated to the 10th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2019)” published in Mediastinum. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/med-2021-01
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-2021-01). The series “Dedicated to the 10th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2019)” was commissioned by the editorial office without any funding or sponsorship. Malgorzata Szolkowska serves as an unpaid editorial board member of Mediastinum from June 2019 to May 2021. Marcin Zielinski serves as an unpaid associate editor-in-chief of Mediastinum from February 2020 to January 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Travis WD, Brambilla E, Burke AP, et al. WHO classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th Ed. Lyon: IARC, 2015.
- Suster S, Moran CA: Thymolipoma. In: Diagnostic Pathology: Thoracic: 2nd Ed. Philadelphia: Elsevier, 2017.
- Weissferdt A, Moran CA. Lipomatous tumors of the anterior mediastinum with muscle differentiation: a clinicopathological and immunohistochemical study of three cases. Virchows Arch 2014;464:489-93. [Crossref] [PubMed]
- Ajaz B, Tran TA, Truong T, et al. Thymolipoma with sebaceous differentiation: a hitherto unreported variant of thymolipoma. Int J Surg Pathol 2013;21:526-30. [Crossref] [PubMed]
- Hudacko R, Aviv H, Langenfeld J, et al. Thymolipoma: clues to pathogenesis revealed by cytogenetics. Ann Diagn Pathol 2009;13:185-8. [Crossref] [PubMed]
- Ströbel P, Hartmann E, Rosenwald A, et al. Corticomedullary differentiation and maturational arrest in thymomas. Histopathology 2014;64:557-66. [Crossref] [PubMed]
- Szolkowska M, Langfort R, Winiarski S, et al. D2-40 Antibody is a Specific Marker for B2 Thymomas. Appl Immunohistochem Mol Morphol 2017;25:445-9. [Crossref] [PubMed]
- Radovich M, Pickering CR, Felau I, et al. The Integrated Genomic Landscape of Thymic Epithelial Tumors. Cancer Cell 2018;33:244-58.e10. [Crossref] [PubMed]
- Moran CA, Rosado-de-Christenson M, Suster S. Thymolipoma: clinicopathologic review of 33 cases. Mod Pathol 1995;8:741-4. [PubMed]
- Toyama T, Mizuno T, Masaoka A, et al. Pathogenesis of thymolipoma: report of three cases. Surg Today 1995;25:86-8. [Crossref] [PubMed]
- Kondo T, Masuya D, Mukai K. Thymolipoma, report of a case suggesting an origin from thymic true hyperplasia. Int J Surg Pathol 2010;18:526-9. [Crossref] [PubMed]
- Kim YK, Shin N, Park WY, et al. Mediastinal thymolipoma with striated myoid cells: report of a peculiar case. Korean J Pathol 2013;47:596-8. [Crossref] [PubMed]
- Ogino S, Franks TJ, Deubner H, et al. Thymohemangiolipoma, a rare histologic variant of thymolipoma: a case report and review of the literature. Ann Diagn Pathol 2000;4:236-9. [Crossref] [PubMed]
- Moran CA, Zeren H, Koss MN. Thymofibrolipoma. A histologic variant of thymolipoma. Arch Pathol Lab Med 1994;118:281-2. [PubMed]
- Kang GH, Han J, Kim TS, et al. Thymofibrolipoma - A Brief Case Report. Korean J Pathol 2010;44:338-40. [Crossref]
- Argani P, de Chiocca IC, Rosai J. Thymoma arising with a thymolipoma. Histopathology 1998;32:573-4. [Crossref] [PubMed]
- Yvorel V, Forest F, Parietti E, et al. B3 thymoma arising within thymolipoma. Pathology 2015;47:702-5. [Crossref] [PubMed]
Cite this article as: Szolkowska M, Blasinska K, Czajkowski W, Zielinski M, Bartczak A, Knetki-Wroblewska M, Kowalski D, Wiśniewski P, Marx A. A thymoma or not a thymoma—that is the question: a case report. Mediastinum 2021;5:38.


