Smoke inhalation injury: a narrative review
Introduction
The term smoke ‘inhalation damage’ describes the inhalation of hot gas and the toxic products of incomplete combustion. This syndrome encompasses three clinical entities: thermal damage to the upper airway, chemical-inflammatory damage to the lower airway, and systemic damage. Variability in the manifestation of this pathology, together with a wide clinical spectrum in terms of its severity, makes it difficult to define and diagnose inhalation damage with certainty in every person exposed to smoke.
A recent meta-analysis showed that a mean of 19.8% patients admitted to hospital with burns have concomitant inhalation damage, and this figure has remained relatively stable in recent decades (1). The studies included in this aforementioned work indicated an overall mortality rate of 10.9%, which reached 20.7% when considering only studies carried out in critical care units. Together, these analyses point towards a significant association between inhalation injury and hospital mortality.
This review discusses our current understanding of smoke inhalation injury. The literature has been reviewed from 2000 to 2020 and the most relevant references were identified. The goal of this review is to comprehensively summarize the pathophysiology, diagnoses, treatment options and current research.
We present the following article in accordance with the Narrative Review reporting checklist (available at: http://dx.doi.org/10.21037/med-21-7).
Pathophysiology
Inhalation damage lesions are classified into three types: (I) heat injuries restricted to upper airway structures, except in the case of steam jet exposure; (II) local chemical irritation throughout the respiratory tract; and (III) systemic toxicity, such as the result of the inhalation of carbon monoxide (CO) or cyanide (HCN).
Upper airway damage
Damage to the upper airway is caused by direct thermal injury or by chemical irritation. Inhalation of air at a temperature exceeding 150 °C causes direct damage to the face, oropharynx, and upper airway. The heat and chemical components in smoke cause immediate damage to the mucosa, although the clinical consequences of this contact do not become apparent until the resulting edema is sufficient to compromise the upper airway. The presence of a skin burn at the neck level magnifies the problem in direct proportion to its extent and depth. In addition, both the fluid required to treat these burns during the initial phase of the injury, as well as the mediators released from these lesions, play a role in airway edema.
Lower airway damage
The nature of this type of injury is not thermal but rather, is of a chemical origin because hot air cools before reaching the carina (except in cases of the inhalation of steam at extremely high temperatures). Smoke-related toxins damage the epithelium and capillary endothelial cells of the airway: mucociliary clearance is altered and bacterial clearance is reduced; edema, inflammation, and bronchospasm can cause airway obstruction; the loss of surfactant facilitates alveolar collapse and atelectasis; and increased capillary permeability magnifies pulmonary and airway edema.
Systemic damage
Systemic damage is produced by the components of the gaseous phase of smoke. In this sense, on the one hand, CO is a colorless and odorless gas that is produced by the incomplete combustion of many materials. CO crosses the alveolocapillary membrane and produces tissue hypoxia through several mechanisms: (I) it displaces oxygen from hemoglobin (Hb) because its affinity for oxygen is 200 times greater than Hb, thus reducing the arterial oxygen content (even though PaO2 and SaO2 measurements may be normal); (II) it shifts the Hb dissociation curve to the left, thereby decreasing oxygen tissue availability; (III) it impairs mitochondrial function and ATP production; (IV) it can bind to skeletal and cardiac muscle where it produces toxic effects; and (V) it has a demyelinating effect on the central nervous system.
On the other hand, HCN is a product that can be generated from the combustion of several different natural or synthetic materials that contain nitrogen. Inhaled aerosolized cyanide is rapidly absorbed by lung tissue where it binds to cytochrome proteins, inhibiting cellular metabolism and production of ATP in tissues. All cells, but especially liver cells, detoxify hydrocyanide by converting it into thiocyanate, which is excreted in urine. This protective system can become saturated in the presence of a large amount of cyanide, especially if the patient is hypovolemic which worsens the metabolism of cyanide and its clearance. In addition to all the above, the process of combustion itself consumes oxygen which reduces the inspired oxygen fraction from the environment at the time of the injury-causing incident. The combination of CO and HCN poisoning, as well as inhalation of oxygen-poor air leads to tissue hypoxia.
Although every level of the respiratory tract, from the oropharynx to the alveoli, are affected by smoke inhalation, the clinical situation of the patient will be influenced by the magnitude of their exposure, the toxicity of the constituents of the smoke they were exposed to, its temperature, oxygen concentration, and smoke concentration, as well as their secondary systemic response to inhalation, which can cause further lung tissue damage.
Diagnosis
At the time of diagnosis, physicians must strive to obtain information about the source of the fire, presence of smoke, duration of patient exposure, whether the exposure location was confined, and the initial neurological status of the patient.
Upper airway damage
Upper airway damage should be suspected in cases of smoke inhalation in closed places, in patients with facial and neck burns with involvement of the lips, nasal vibrissae, and oropharyngeal mucosa, and when patients present progressive hoarseness and a cough accompanied by carbonaceous sputum. Indeed, the abovementioned clinical picture can evolve into the presentation of symptoms consistent with airway obstruction. However, increasingly labored breathing with stridor and cyanosis will not appear until critical narrowing of the airway is already present. Moreover, the course of airway and skin burn edema processes will develop in parallel to the above, adding to the combined internal and external anatomical distortions. The oropharynx of all burn patients should be examined for soot or evidence of chemical or thermal damage. In this sense, direct laryngoscopy is a useful, quick, and easy method. Damage to the airway mucosa is manifested by the presence of edema, erythema, and ulceration. Because edema progresses over 24 hours, repeated evaluations will be required if an inhalation damage injury is identified and intubation was not performed. Fibroscopy is also useful both in suspected airway damage and during pulmonary evaluations.
Lower airway damage
The diagnosis of lower airway damage is based on the clinical course of the pathology rather than on initial findings in the patient. The presence of soot may be evident in secretions for days and is an indicator of smoke exposure, but in itself is insufficient to establish a diagnosis or the severity of inhalation damage. Rhonchi and wheezing may appear during the inflammation process. Furthermore, continuous coughing, bronchospasm, and bronchorrhea can lead to fatigue and hypoventilation. The most serious clinical consequences are related to lung infections or to airway obstruction and intrapulmonary shunt, both of which usually develop in the few days after the exposure. Unfortunately, in the days immediately after suspected inhalation damage, lung damage cannot be detected by chest radiography. Thus, while the use of a bronchoscope during the initial diagnosis of inhalation damage is clearly helpful, it rarely leads to a specific therapeutic strategy.
Systemic damage
The clinical manifestations of CO intoxication appear when carboxyhemoglobin (COHb) levels exceed 15%, with symptoms of tissue hypoxia, among which neurological deterioration and myocardial dysfunction especially stand out. No particular combination of symptoms can confirm or exclude the diagnosis of CO poisoning; the clinical diagnosis must be confirmed with lab results indicating an elevated COHb level. COHb can be measured by performing spectrophotometry on blood drawn either at the scene of the accident or obtained during the patient evaluation in the Emergency Department.
Fingertip pulse co-oximeter technology has been available since 2005, although it is less accurate, and its readings must be confirmed. Low blood oxygen saturation levels determined in the hospital setting do not always indicate minimal smoke inhalation exposure, because the administration of oxygen from the time treatment starts can help normalize these levels during patient transfer. Thus, the use of total blood carbon monoxide (TBCO) measurements as an alternative biomarker for CO poisonings has been proposed because TBCO determination results may be more valid and stable, even under non-optimal storage conditions. Additionally, TBCO can be used to predict COHb in cases where sample degradation hinders optical measurement (2).
HCN poisoning depends on the concentration of cyanides in the smoke and presents as metabolic acidosis and clouding of consciousness in severe cases. The persistence of metabolic acidosis in a burn patient with an adequate resuscitation volume and cardiac output suggests CO or HCN poisoning. When considering intoxication, it is useful to measure the acid-base and plasma lactate balances, although determination of their levels is not currently clinically available (3).
Treatment
Upper airway damage
When faced with potential upper airway damage, it is important to decide whether the airway must be protected by orotracheal intubation or whether it can be safely managed without it. Physicians should not wait for signs of obstruction to appear at this level before making this decision, and if there is any doubt that the edema is progressing, it is safer to intubate.
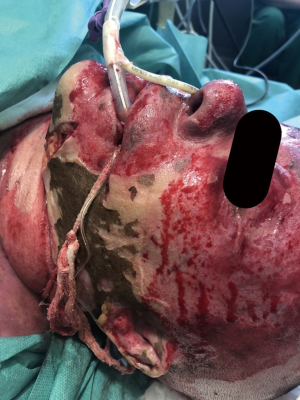
A publication by Demling (4) described three categories of patients at risk of upper airway compromise: (I) those with hot smoke inhalation without facial burns. In these cases, if there is no evidence of severe upper airway edema, this group can be carefully monitored; (II) patients with significant oral burns without smoke inhalation. It is usually difficult to control secretions if edema progresses in the patients in this category and so, in these cases, early intubation is a safe practice because the anatomical distortion of the mouth can make subsequent intubation difficult; (III) individuals with hot smoke inhalation with extensive burns on their face and neck (Figure 1). These patients invariably require intubation.
If they are hemodynamically stable, both intubated and non-intubated patients should be maintained in a semi-upright position to minimize the process of facial and airway edema. If intubation is performed, the tube must be very securely fixed to prevent accidental extubation. Tracheostomy is not initially indicated as an elective technique, although it may be the only possible strategy in an emergency in non-intubatable patients.
Lower airway damage
Adequate oxygenation must be maintained, and bronchial hygiene should be facilitated in cases of lower airway damage. Some patients may also be receiving non-invasive mechanical ventilation or high-flow oxygen therapy. Invasive mechanical ventilation may be necessary in patients with labored breathing or compromised gas exchange. High frequency oscillatory ventilation (HFOV) has not been shown to have an obvious benefit in patients with respiratory distress secondary to inhalation injury and is not considered a first-line therapy. Similarly, to date, evidence for the use of high frequency percussive ventilation (HFPV) is scant and equivocal. Consensus recommendations for mechanical ventilation, as well as strategies aimed at preventing ventilator-associated pneumonia (elevating the patient’s head, maintaining endotracheal cuff pressure, frequently changing the posture of the patient, and oral hygiene), are both useful in this context.
The administration of bronchodilators is useful in patients presenting bronchospasm. Bronchoscopy can improve lung hygiene and prognosis by removing secretions and detached epithelial cells (5). Moreover, positioning in the prone position may be helpful in patients suffering from severe respiratory distress. In refractory cases, inhaled nitric oxide causes specific vasodilation in well-ventilated segments of the lung and can improve oxygenation and pulmonary hemodynamics, although, to date, it has not been related to increased survival rates.
Some studies have suggested the efficacy of aerosolized heparin for the treatment of lower airway damage, particularly in pediatric patients, although no evidence for this has yet been provided in clinical trials and its use has not been standardized. Although recent data have confirmed that nebulized tocopherol can produce good results in experimental studies, it is not yet used in clinical settings. Similarly, given the absence of well-designed studies supporting the use of steroids in patients with respiratory distress syndrome secondary to inhalation damage, their routine use for treatment is not currently recommended. Antioxidant aerosols, cytokine inhibitors, and neutrophil adhesion inhibitors have also been tested in experimental studies and were associated with a reduction in alveolar and mucosal edema and atelectasis. Moreover, extracorporeal oxygenation membranes can be used as a rescue treatment in refractory situations when this resource is available.
Future research aimed at addressing inhalation damage should focus on determining the underlying molecular and cellular mechanisms of this pathology, while new treatments are being developed within the fields of regenerative medicine and bioengineering (6).
Systemic damage
Treatment of patients with systemic damage begins by removing the victim from exposure as soon as possible and immediately administering high-flow oxygen.
The goal of oxygen therapy in patients with CO poisoning is to displace CO from Hb. The COHb concentration is reduced approximately 50% every 20 minutes when 100% oxygen is administered. To properly treat CO poisoning, it is important to determine the COHb concentration as soon as possible and to administer oxygen therapy to any patient with COHb levels below 10%. Endotracheal intubation with the provision of 100% oxygen with mechanical ventilation is indicated in patients with neurological or hemodynamic deterioration and elevated COHb.
Hyperbaric oxygen (at 2–3 atm) produces faster CO displacement and may be beneficial in cases with prolonged exposure when CO displacement from the cytochrome system is more difficult. The disadvantage is that patients with hemodynamic and pulmonary instability must be transferred to a center with a hyperbaric chamber. Thus, this option would be best considered in patients with a severely compromised neurological component, COHb exceeding 50%, without extensive burns or severe lung damage, and whose symptomology does not improve in response to high-flow oxygen.
The treatment of HCN poisoning is aimed at cardiopulmonary optimization, which is usually sufficient because the liver clears the cyanide from the circulation. Hydroxocobalamin should be used as early as possible (7) in patients exposed to smoke (with the presence of traces of soot in their mouth, pharynx, or sputum), with neurological disorders (confusion, coma, agitation, or convulsions), or who present bradypnea or respiratory or cardiorespiratory arrest, shock or hypotension, lactate ≥7.5 mmol/L, or metabolic acidosis. The individual use of amyl nitrite, sodium nitrite, or sodium thiosulfate is useful for treating cyanide poisoning. However, nitrites induce methemoglobin and so they should be used with caution (8).
Acknowledgments
To Dr. Simon R. Turner, for offering me the opportunity to review this entity for the scientific community.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Simon R. Turner) for the series “Traumatic Injuries of the Mediastinum” published in Mediastinum. The article has undergone external peer review.
Reporting Checklist: The author has completed the Narrative Review reporting checklist. Available at: http://dx.doi.org/10.21037/med-21-7
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/med-21-7). The series “Traumatic Injuries of the Mediastinum” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Galeiras R, Seoane-Quiroga L, Pértega-Díaz S. Prevalence and prognostic impact of inhalation injury among burn patients: A systematic review and meta-analysis. J Trauma Acute Care Surg 2020;88:330-44. [Crossref] [PubMed]
- Oliverio S, Varlet V. New strategy for carbon monoxide poisoning diagnosis: Carboxyhemoglobin (COHb) vs Total Blood Carbon Monoxide (TBCO). Forensic Sci Int 2020;306:110063 [Crossref] [PubMed]
- Anseeuw K, Delvau N, Burillo-Putze G, et al. Cyanide poisoning by fire smoke inhalation: a European expert consensus. Eur J Emerg Med 2013;20:2-9. [Crossref] [PubMed]
- Demling RH. Smoke inhalation injury. New Horiz 1993;1:422-34. [PubMed]
- Dries DJ, Endorf FW. Inhalation injury: epidemiology, pathology, treatment strategies. Scand J Trauma Resusc Emerg Med 2013;21:31. [Crossref] [PubMed]
- Guo B, Bai Y, Ma Y, et al. Preclinical and clinical studies of smoke-inhalation-induced acute lung injury: update on both pathogenesis and innovative therapy. Ther Adv Respir Dis 2019;13:1753466619847901 [Crossref] [PubMed]
- Fortin JL, Giocanti JP, Ruttimann M, et al. Prehospital administration of hydroxocobalamin for smoke inhalation-associated cyanide poisoning: 8 years of experience in the Paris Fire Brigade. Clin Toxicol (Phila) 2006;44:37-44. [Crossref] [PubMed]
- Betten DP, Vohra RB, Cook MD, et al. Antidote use in the critically ill poisoned patient. J Intensive Care Med 2006;21:255-77. [Crossref] [PubMed]
Cite this article as: Galeiras R. Smoke inhalation injury: a narrative review. Mediastinum 2021;5:16.