Targeted therapies for unresectable stage III non-small cell lung cancer
Introduction
Non-small cell lung cancer (NSCLC) represents 85% of all primary lung cancers, and approximately 20% to 25% of these patients present with locally advanced disease (stage III) (1). Of note, the proportion of new lung cancer cases presenting as stage III has decreased steadily, from 28.6% in 1998 to 26.6% in 2006, possibly as a consequence of an increase in stage IV NSCLC after the year 2000 (from 35.7% to 39.4%). This data probably reflects the widespread adoption of fluor-deoxyglucose positron emission tomography (FDG-PET) scans and magnetic resonance imaging (MRI) of the brain leading to a better radiological assessment and staging (2). Concurrent chemoradiotherapy (cCT-RT) remains the standard treatment approach for patients with unresectable stage III NSCLC and good performance status (1). However, before 2017 the prognosis for these patients was still dismal, with a median overall survival (OS) ranging from 19.6 months to 28.7 months (3-5), and a 5-year OS rate ranging from 15% to 32.1% in more recent series (1,5). Different strategies have been tested with the aim to improve the outcome in this population. Radiation dose escalation and the addition of cetuximab to cCT-RT treatment did not provide any survival benefit compared with the standard approach while increasing treatment-related toxicities (5). Likewise, maintenance treatment with vaccination after chemo-radiotherapy did not improve the survival in the whole population compared with placebo (6). Finally, the addition of bevacizumab was not recommended given the lack of an efficacy signal and the substantial risk of esophageal toxicity (7).
After 2017, the phase III PACIFIC trial shifted the treatment paradigm in unresectable stage III NSCLC patients. The trial reported that consolidation treatment with one year of durvalumab after cCT-RT compared with placebo significantly improved the progression free survival (PFS: 17.2 vs. 5.6 months, Hazard Ratio, HR =0.51, 95% CI: 0.41-63, P<0.0001) (8) and the OS (47.5 vs. 29.1 months, HR 0.71, 95% CI: 0.57–0.88) with a 4-year OS of 49.6% vs. 36.3%, respectively (9). The benefit of durvalumab occurred without detrimental effect on patient-reported outcomes (10). Programmed cell death-ligand 1 (PD-L1) status was not mandatory for inclusion in the PACIFIC trial, and PD-L1 status was unknown in 37% of all randomized patients. A prespecified exploratory analysis assessed the benefit of durvalumab according to PD-L1 expression ≥25% or <2%% (by SP263 IHC assay) and confirmed the benefit regardless of PD-L1 expression level. However, a post-hoc analysis requested by the European Medicines Agency (EMA) with a PD-L1 expression-level cut-off of 1% suggested that PFS benefit with durvalumab occurred across all subgroups, but an OS benefit was found only in those tumors with a PD-L1 ≥1% tumors (9,11). Based on these data, the FDA approved consolidation durvalumab as a new standard of care regardless of PD-L1 expression in February 2018, whereas the EMA approval in September 2018 was limited to the PD-L1 ≥1% tumors. Likewise, the recent phase II LUN 14-179 clinical trial (12) has reported that consolidation pembrolizumab after cCT-RT did also improve the outcome in comparison with historical controls, endorsing the immune-strategy in this setting. Finally, preliminary data of trials evaluating immunotherapy concurrent with cCT-RT are also promising (13,14).
This new standard therapeutic approach put into question whether all patients with locally advanced NSCLC may obtain benefit of an immunotherapy consolidation strategy after cCT-RT. This is especially relevant for oncogenic addicted tumors. In the metastatic setting, this subpopulation of lung tumors did obtain only a limited efficacy with immune checkpoint inhibitors as monotherapy (15), and in locally advanced disease, the PACIFIC trial did not improve the outcome with durvalumab compared with placebo among the 6% of epidermal growth factor receptor (EGFR)-mutant tumors enrolled in the trial (9). Indeed, in real world data, consolidation with durvalumab appears to be less efficacious in patients with ERBB2/EGFR mutant tumors (tumors harboring ERBB2/EGFR mutation had a significantly shorter disease free survival compared to the EGFR/ERBB2 wildtype tumors, 7.5 months vs. not reached, P=0.04) (16). Of note, a retrospective analysis of 37 patients with unresectable stage III EGFR-mutated NSCLC assessed the role of consolidation strategy either with durvalumab or EGFR tyrosine kinase inhibitor (TKI) after completion of cCT-RT. Out of these 37 patients, 13 initiated durvalumab a median of 20 days after cCT-RT completion. Two patients completed 12 months of treatment, with five patients discontinuing durvalumab due to progression and five due to immune-related adverse events (irAEs). Of 24 patients who completed cCT-RT without durvalumab 16 completed CRT alone and 8 completed cCT-RT with induction or consolidation EGFR TKI. Median PFS was 10.3 months in patients who received cCT-RT and durvalumab versus 6.9 months with cCT-RT alone (log-rank P=0.993). The cCT-RT and EGFR TKI was associated with a significantly longer median PFS (26.1 months) compared to cCT-RT and durvalumab or CRT alone (log-rank P=0.023) (17). Similarly, the REFRACT study, a pooled retrospective analyses including patients with locally advanced NSCLC and EGFR mutation, reported that radiotherapy plus EGFR TKI with or without chemotherapy was associated with improved PFS relative to chemo-radiotherapy (HR =0.42, 95% CI: 0.29–0.61, P<0.001) and OS (HR =0.60, 95% CI: 0.37–0.99, P=0.045), as well as improved PFS compared to EGFR TKI as monotherapy (HR =0.65, 95% CI: 0.47–0.90, P=0.008) and marginally better OS relative to TKI (HR =0.67, 95% CI: 0.41–1.11, P=0.12). The improved outcome with the addition of EGFR TKI to standard chemo-radiotherapy could be related to better local and distant control. These data may suggest the increased risk of toxicity among EGFR-mutant tumors with consolidation treatment with durvalumab and the potential role of exploring personalized approaches with TKI against oncogenic drivers in this setting, being an area of ongoing and for future research.
Personalized treatment in locally-advanced disease
The discovery of targetable oncogenic drivers in advanced NSCLC (18) and the development of targeted therapies against these targets, mainly TKI and antibody drug conjugated drugs, have revolutionized the therapeutic strategy in this setting (19,20). This strategy provides a personalised treatment approach in advanced NSCLC contributing to an improvement in the OS (21), as well as a reduction in lung cancer mortality in most recent years (22). Of note, genomic alterations reported in advanced tumours are also found in early stage lung cancers (18), challenging the role of personalised treatment in unresectable stage III NSCLC.
EGFR mutation
In the metastatic setting, the prevalence of EGFR mutations is around 10–20% in the Caucasian population with adenocarcinoma but much higher in Asian populations (~50%). Around 90% of the most common EGFR mutations comprise deletions in exon 19 and the L858R substitution mutation in exon 21. These mutations confer sensitivity to EGFR TKI (19,20). In EGFR-mutant advanced NSCLC, first-generation (gefitinib and erlotinib) and second-generation EGFR TKI (afatinib and dacomitinib) resulted in an improved outcome compared with the standard of care (19,20). Recently, the phase III FLAURA trial reported that osimertinib, a third-generation EGFR TKI, improved the PFS and OS compared with first-generation EGFR TKI with better intracranial activity. As a result, osimertinib became the preferred upfront strategy in this subset of lung adenocarcinomas (23). Likewise, osimertinib has once again shifted the treatment paradigm with the phase III ADAURA results, this time in completely resected stage I-IIIA NSCLC with common EGFR mutations reporting a significant improvement in disease free survival compared with placebo after optional adjuvant chemotherapy (24).
Some studies have reported that EGFR mutation prevalence in locally advanced NSCLC ranges from 10% to 30% (25–27), probably as a consequence of the different ethnicity population tested. The outcome of chemo-radiotherapy in unresectable stage III disease harboring oncogenic drivers remains controversial. Some authors (26,28) have reported that median PFS after radical chemo-radiotherapy was significantly shorter in stage III EGFR-mutant tumors compared with wild-type tumors (9.6 vs. 12.0 months; multivariate HR 2.0, 95% CI: 0.9–4.2, P=0.003), although no differences in OS were reported (29.4 vs. 23.4 months, P=0.21) (26). In contrast, other authors have reported longer median OS in EGFR-mutant tumors compared with wild-type tumors, although the difference was not statistically significant (29,30). Meanwhile, the frequency of distant metastases in EGFR-mutant tumors after cCT-RT was higher than in the wild type tumors or tumors with other oncogenic alterations (28,29,31). This was especially found for brain metastases with a cumulative incidence of brain metastases at 3-years and 5 years of 33% and 44%, respectively (31). These data along with data from a systematic review and meta-analysis suggest that stage III EGFR-mutant tumors have shorter PFS on cCT-RT than wild type, mainly because of distant metastasis relapse, especially brain metastases, regardless of better local control (32). Based on the efficacy of EGFR TKI in the metastatic setting and in early-stage EGFR-mutant NSCLC, especially with osimertinib, the EGFR TKI strategy was started to be tested in unresectable stage III EGFR-mutant NSCLC with the aim of extending the positive results in this setting and change the natural history of this disease.
A retrospective study assessed whether EGFR TKI (n=177) could substitute the cCT-RT (n=22) in stage III EGFR-mutant NSCLC patients. The study did not find differences in OS (HR 0.71, 95% CI: 0.34–1.47) or lung cancer-specific survival (HR 0.65, 95% CI: 0.31–1.35), yielding a 5-year OS of 30% and 25%, respectively (33). The limited number of patients and the retrospective nature of this analysis do not lead to obtain firm conclusions whether EGFR TKI alone may be the preferred treatment option instead of the cCT-RT in EGFR-mutant unresectable stage III NSCLC.
Preclinical studies have suggested that EGFR-mutant NSCLC cells have a predominantly radiosensitive phenotype and EGFR TKI may have a radiosensitizing effect (34,35). These data provide rationale to assess the application of EGFR TKI either in combination with radiotherapy or as a consolidation or maintenance strategy after cCT-RT (Table 1). However, it is relevant to mention that a recent modeling study predicted that targeted induction therapies before chemo-radiotherapy may render adjuvant targeted therapy less effective due to proliferation of drug-resistant cancer cells when using very long induction periods (45).
Table 1
| Strategy | References |
|
|---|---|---|
| EGFR TKI |
( |
Yes |
| EGFR TKI + RT |
( |
Yes |
| EGFR TKI + cCT-RT/sCT-RT | ( |
No |
| EGFR TKI + RT | ( |
No |
| NCT04636593 | Yes | |
| EGFR TKI → EGFR TKI + cCT-RT | ( |
Yes |
| EGFR TKI → cCT-RT | ( |
Yes |
| RTOG 1306 (NCT01822496) | Yes | |
| cCT-RT → EGFR TKI | ( |
No |
| ( |
Yes | |
| NCT03396185 | Yes |
cCT-RT, concurrent chemotherapy-radiotherapy; sCT-RT, sequential chemotherapy-radiotherapy.
The randomized phase II RECEL (NCT0174908) screened 252 patients and enrolled 41 unresectable EGFR-mutant stage III NSCLC patients, who were randomized to erlotinib for 2 years plus radiotherapy or cCT-RT. In the erlotinib arm the PFS significantly improved compared with the cCT-RT arm (27.9 vs. 6.4 months, HR 0.053, 95% CI: 0.006–0.463, P<0.001), with the same incidence of adverse events (AEs, grade ≥1, 86.7%, 13/15) being the most common AEs grade ≥3 the rash (20%) and hematological toxicity (27%) (36). This data provides rationale for the role of EGFR TKI plus radiotherapy in stage III in EGFR-mutant tumors, but warrants further evaluation in a phase III clinical trial. A similar strategy is being explored in the ongoing single arm phase II WJOG6911L study with gefitinib (46).
The addition of EGFR TKI to a chemo-radiotherapy strategy has been assessed in phase II trials, but most of these trials included patients either with wild-type or unknown EGFR status. The CALGB 30106 trial assessed the addition of gefitinib to sequential or cCT-RT in 63 unresectable stage III NSCLC patients. In this trial all patients received 2 cycles of induction chemotherapy plus gefitinib followed by radiotherapy plus gefitinib in poor performance status patients, or cCT-RT plus gefitinib in good-risk patients. Although the toxicity was not increased, compared with historical data, the median OS data was very disappointing (19 and 13 months in the poor-risk and good-risk group, respectively). There were no differences in PFS (P=0.87) or OS (P=0.88) among 13 EGFR-mutant tumors compared with wild-type tumors (37). Similar outcomes were reported in another phase II trial (CALGB30605) (40) assessing erlotinib plus radiotherapy after 2 cycles of induction chemotherapy in poor-risk stage III NSCLC patients (PFS: 11 months, OS: 17 months). However, no patients with EGFR mutation were identified in this trial. In contrast, two phase II trials reported promising survival data either with gefitinib and concurrent thoracic radiotherapy after induction chemotherapy (38) or erlotinib plus cCT-RT (39), reaching a 2-year OS rate of ~65%. These findings may suggest a survival benefit with EGFR TKI in this setting, although EGFR mutation was not mandatory and only 5 EGFR-mutant patients were included in the former study (39), limiting the potential conclusions in this subset of lung adenocarcinomas. Among 12 EGFR-mutant unresectable stage III NSCLC patients, induction treatment with erlotinib followed by either cCT-RT plus erlotinib (N=7) or by cCT-RT (N=5) did not report differences either in OS (39.3 vs. 31.2 months, P=0.442) or in PFS (11.6 vs. 8.1 months, P=0.134). Although EGFR-mutant tumors had better OS than wild-type EGFR tumors (74.8 vs. 25.3 months, P=0.034) probably related to subsequent EGFR TKI therapies at the time of progression, the distant failure rate was higher in EGFR-mutant tumors compared with wild-type tumors (63% vs. 42%, P=0.463). This was especially found for brain metastases, as these were the more common site of the first relapse in the EGFR-mutant group, even though there was no statistical significant difference between groups (46% vs. 18%, P=0.070) (41). This data may suggest that EGFR TKIs with higher intracranial penetration is necessary in this setting if we want to change the natural history of this disease.
Finally, two phase 2 clinical trials assess the role of induction EGFR TKI before cCT-RT in EGFR mutant stage III tumors, the LOGIK0902/OLCSG0905 intergroups study with gefitinib (42), and the RTOG 3106 (NCT01822496) with induction erlotinib followed by cCT-RT or only CT-RT. The later trial has terminated due to lack of accrual.
Maintenance strategy with EGFR TKI in an unselected population was assessed in the phase III SWOG S0023 trial (43). Patients who did not progress after cCT-RT with platinum and etoposide and three cycles of consolidation with docetaxel were randomized to maintenance treatment with gefitinib or placebo for 5 years. The study was closed prematurely as after a median follow-up of 27 months, median OS was 23 months with gefitinib, whereas it reached 35 months with placebo (P=0.013). The decreased survival was primarily due to disease progression rather than treatment toxicity, as toxic death rate was not different from placebo (2% vs. 0%). It is important to notice that this trial did not select patients according to EGFR mutation status. Perhaps selectively treating patients only with EGFR mutations with gefitinib may lead to different outcomes.
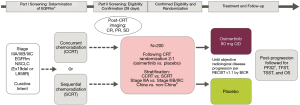
Despite limited data about EGFR TKI efficacy for patients with stage III EGFR-mutant NSCLC, evidence has suggested that these patients have inferior distant control following platinum-based CRT compared with those who have EGFR wild-type disease, especially central nervous system (CNS) control (32), highlighting the need for targeted therapy in patients with these disease features. The phase 3 LAURA clinical trial (NCT03521154) is currently enrolling unresectable EGFR-mutant stage III NSCLC patients to explore the efficacy and safety of osimertinib compared with placebo (2:1) until progression as maintenance therapy in patients without progression after concurrent or sequential chemoradiation (44). The primary end point is PFS per RECIST 1.1 according to blinded independent central review (BICR); and secondary end points include CNS PFS, PFS by mutational status, OS, safety, and tolerability (Figure 1). Moreover, almonertinib, a new third generation EGFR TKI is being tested in a phase II trial combined with thoracic radiotherapy in stage III NSCLC with an activating EGFR mutation. Primary endpoint is incidence of grade 3 or higher radiation pneumonitis within 6 months of radiotherapy (NCT04636593). Similarly, the first-generation EGFR TKI icotinib is being tested in a single arm phase II trial as maintenance therapy after sequential or cCT-RT in the same patient population (NCT03396185). Primary endpoint is OS. Furthermore, a retrospective Chinese cohort of stage III and EGFR-mutant NSCLC patients is assessing the best treatment approach in this setting: chemoradiotherapy, chemoradiotherapy plus EGFR TKI, or EGFR TKI alone (NCT04304638). The results of this trial may help to state the role of EGFR TKI in locally advanced setting
ALK rearrangement
In locally advanced disease, the prevalence of ALK rearrangement ranges from 2% to 8% (25,26,46). Poorer PFS has been reported in ALK-positive tumors after chemoradiotherapy compared with wild type (6 vs. 12 months, HR 2.8, 95% CI: 1.5–5, P=0.003). Based on the efficacy of ALK TKI in the metastatic setting (47,48) it is logic to explore the role of these drugs in the locally advanced setting. For now, only the RTOG 3106 (NCT01822496) has randomized stage III ALK-positive NSCLC patients to receive either induction with crizotinib for three months followed by cCT-RT or only cCT-RT. This trial has terminated due to the poor accrual.
Conclusions
The current evidence does not support the use of TKI in oncogenic addicted tumors in stage III. However, the limited efficacy of chemo-radiotherapy for these tumors (EGFR/ALK) and risk of distant metastases support to further explore the use of TKI in this setting. Another challenge today is whether oncogenic addicted tumors should or should not receive consolidation immunotherapy after cCT-RT based on limited efficacy, as well as the finding that sequential immunotherapy followed by a TKI may increase toxicity. Therefore, the optimal strategy in stage III NSCLC patients with oncogenic drivers deserves further evaluation.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Giuseppe Banna and Alfredo Addeo) for the series “Changes in management of mediastinal tumours following the surge of COVID-19 pandemic” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-21-8). The series “Changes in management of mediastinal tumours following the surge of COVID-19 pandemic” was commissioned by the editorial office without any funding or sponsorship. JR reports personal fees and other from MSD, other from BOEHRINGER, other from PFIZER, personal fees and other from OSE IMMUNOTHERAPEUTICS, other from BMS, other from ASTRAZENECA, other from ROCHE, outside the submitted work. LELH reports other from boehringer ingelheim, other from BMS, other from Roche Genentech, other from BMS, grants from Roche Genentech, grants from Boehringer Ingelheim, other from AstraZeneca, personal fees from Quadia, grants from Astra Zeneca, other from Eli Lilly, other from Roche Genentech, other from Pfizer, other from MSD, other from Takeda, non-financial support from AstraZeneca, non-financial support from Novartis, non-financial support from BMS, non-financial support from MSD /Merck, non-financial support from GSK, non-financial support from Takeda, non-financial support from Blueprint Medicines, non-financial support from Roche Genentech, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects for the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181-90. [Crossref] [PubMed]
- Morgensztern D, Ng SH, Gao F, et al. Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J Thorac Oncol 2010;5:29-33. [Crossref] [PubMed]
- Steuer CE, Behera M, Ernani V, et al. Comparison of Concurrent Use of Thoracic Radiation With Either Carboplatin-Paclitaxel or Cisplatin-Etoposide for Patients With Stage III Non-Small-Cell Lung Cancer: A Systematic Review. JAMA Oncol 2017;3:1120-9. [Crossref] [PubMed]
- Senan S, Brade A, Wang LH, et al. PROCLAIM: Randomized Phase III Trial of Pemetrexed-Cisplatin or Etoposide-Cisplatin Plus Thoracic Radiation Therapy Followed by Consolidation Chemotherapy in Locally Advanced Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:953-62. [Crossref] [PubMed]
- Bradley JD, Hu C, Komaki RR, et al. Long-Term Results of NRG Oncology RTOG 0617: Standard- Versus High-Dose Chemoradiotherapy With or Without Cetuximab for Unresectable Stage III Non-Small-Cell Lung Cancer. J Clin Oncol 2020;38:706-14. [Crossref] [PubMed]
- Butts C, Socinski MA, Mitchell PL, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:59-68. [Crossref] [PubMed]
- Socinski MA, Stinchcombe TE, Moore DT, et al. Incorporating bevacizumab and erlotinib in the combined-modality treatment of stage III non-small-cell lung cancer: results of a phase I/II trial. J Clin Oncol 2012;30:3953-9. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Faivre-Finn C, Vicente D, Kurata T, et al. Durvalumab after chemoradiotherapy in stage III NSCLC: 4-year survival update from the phase III PACIFIC trial. Ann Oncol 2020;31:S1178-9. [Crossref]
- Hui R, Özgüroğlu M, Villegas A, et al. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study. Lancet Oncol 2019;20:1670-80. [Crossref] [PubMed]
- Paz-Ares L, Spira A, Raben D, et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann Oncol 2020;31:798-806. [Crossref] [PubMed]
- Durm GA, Jabbour SK, Althouse SK, et al. A phase 2 trial of consolidation pembrolizumab following concurrent chemoradiation for patients with unresectable stage III non-small cell lung cancer: Hoosier Cancer Research Network LUN 14-179. Cancer 2020;126:4353-61. [Crossref] [PubMed]
- Peters S, Felip E, Dafni U, et al. Progression-free and overall survival for concurrent nivolumab with standard concurrent chemo-radiotherapy in locally advanced stage IIIA/B NSCLC: Results from the European Thoracic Oncology Platform NICOLAS phase II trial (ETOP 6-14). J Thorac Oncol 2021;16:278-88. [Crossref] [PubMed]
- Lin SH, Lin Y, Yao L, et al. Phase II Trial of Concurrent Atezolizumab With Chemoradiation for Unresectable NSCLC. J Thorac Oncol 2020;15:248-57. [Crossref] [PubMed]
- Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 2019;30:1321-8. [Crossref] [PubMed]
- Hellyer JA, Aredo JV, Das M, et al. Role of Consolidation Durvalumab in patients with EGFR and HER2 Mutant Unresectable Stage III NSCLC. J Thorac Oncol 2021;16:868-72. [Crossref] [PubMed]
- Aredo JV, Mambetsariev I, Hellyer JA, et al. Durvalumab for Stage III EGFR-Mutated Non-Small Cell Lung Cancer After Definitive Chemoradiotherapy. J Thorac Oncol 2021;16:1030-41. [Crossref] [PubMed]
- Skoulidis F, Heymach JV. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat Rev Cancer 2019;19:495-509. [Crossref] [PubMed]
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv192-237. [Crossref]
- Available online: https://www.esmo.org/guidelines/lung-and-chest-tumours/clinical-practice-living-guidelines-metastatic-non-small-cell-lung-cancer
- Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016;387:1415-26. [Crossref] [PubMed]
- Howlader N, Forjaz G, Mooradian MJ, et al. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N Engl J Med 2020;383:640-9. [Crossref] [PubMed]
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2020;382:41-50. [Crossref] [PubMed]
- Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:1711-23. [Crossref] [PubMed]
- Ryan KJ, Skinner KE, Fernandes AW, et al. Real-world treatment patterns among patients with unresected stage III non-small-cell lung cancer. Future Oncol 2019;15:2943-53. [Crossref] [PubMed]
- Boros A, Lacroix L, Lacas B, et al. Prognostic value of tumor mutations in radically treated locally advanced non-small cell lung cancer patients. Oncotarget 2017;8:25189-99. [Crossref] [PubMed]
- Akamatsu H, Kaira K, Murakami H, et al. The impact of clinical outcomes according to EGFR mutation status in patients with locally advanced lung adenocarcinoma who received concurrent chemoradiotherapy. Am J Clin Oncol 2014;37:144-7. [Crossref] [PubMed]
- Tanaka K, Hida T, Oya Y, et al. EGFR Mutation Impact on Definitive Concurrent Chemoradiation Therapy for Inoperable Stage III Adenocarcinoma. J Thorac Oncol 2015;10:1720-5. [Crossref] [PubMed]
- Ishihara M, Igawa S, Sasaki J, et al. Evaluation of concurrent chemoradiotherapy for locally advanced NSCLC according to EGFR mutation status. Oncol Lett 2017;14:885-90. [Crossref] [PubMed]
- Lim YJ, Chang JH, Kim HJ, et al. Superior Treatment Response and In-field Tumor Control in Epidermal Growth Factor Receptor-mutant Genotype of Stage III Nonsquamous Non-Small cell Lung Cancer Undergoing Definitive Concurrent Chemoradiotherapy. Clin Lung Cancer 2017;18:e169-78. [Crossref] [PubMed]
- Mitra D, Chen YH, Li R, et al. EGFR mutant locally advanced non-small cell lung cancer is at increased risk of brain metastasis. Clin Transl Radiat Oncol 2019;18:32-8. [Crossref] [PubMed]
- Qin Q, Peng B, Li B. The impact of epidermal growth factor receptor mutations on the efficacy of definitive chemoradiotherapy in patients with locally advanced unresectable stage III non-small cell lung cancer: a systematic review and meta-analysis. Expert Rev Anticancer Ther 2019;19:533-9. [Crossref] [PubMed]
- Hsia TC, Liang JA, Li CC, et al. Comparative effectiveness of concurrent chemoradiotherapy versus EGFR-tyrosine kinase inhibitors for the treatment of clinical stage IIIb lung adenocarcinoma patients with mutant EGFR. Thorac Cancer 2018;9:1398-405. [Crossref] [PubMed]
- Anakura M, Nachankar A, Kobayashi D, et al. Radiosensitivity Differences between EGFR Mutant and Wild-Type Lung Cancer Cells are Larger at Lower Doses. Int J Mol Sci 2019;20:3635. [Crossref] [PubMed]
- Zhuang HQ, Sun J, Yuan ZY, et al. Radiosensitizing effects of gefitinib at different administration times in vitro. Cancer Sci 2009;100:1520-5. [Crossref] [PubMed]
- Xing L, Wu G, Wang L, et al. A multicenter, randomized, open-label, phase II trial of erlotinib versus etoposide plus cisplatin with concurrent radiotherapy in unresectable stage III non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) activating mutation. J Clin Oncol 2017;35:8531. [Crossref]
- Ready N, Jänne PA, Bogart J, et al. Chemoradiotherapy and gefitinib in stage III non-small cell lung cancer with epidermal growth factor receptor and KRAS mutation analysis: cancer and leukemia group B (CALEB) 30106, a CALGB-stratified phase II trial. J Thorac Oncol 2010;5:1382-90. [Crossref] [PubMed]
- Niho S, Ohe Y, Ishikura S, et al. Induction chemotherapy followed by gefitinib and concurrent thoracic radiotherapy for unresectable locally advanced adenocarcinoma of the lung: a multicenter feasibility study (JCOG 0402). Ann Oncol 2012;23:2253-8. [Crossref] [PubMed]
- Komaki R, Allen PK, Wei X, et al. Adding Erlotinib to Chemoradiation Improves Overall Survival but Not Progression-Free Survival in Stage III Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2015;92:317-24. [Crossref] [PubMed]
- Lilenbaum R, Samuels M, Wang X, et al. A phase II study of induction chemotherapy followed by thoracic radiotherapy and erlotinib in poor-risk stage III non-small-cell lung cancer: results of CALGB 30605 (Alliance)/RTOG 0972 (NRG). J Thorac Oncol 2015;10:143-7. [Crossref] [PubMed]
- Lee Y, Han JY, Moon SH, et al. Incorporating Erlotinib or Irinotecan Plus Cisplatin into Chemoradiotherapy for Stage III Non-small Cell Lung Cancer According to EGFR Mutation Status. Cancer Res Treat 2017;49:981-9. [Crossref] [PubMed]
- Hotta K, Sasaki J, Saeki S, et al. Gefitinib Combined With Standard Chemoradiotherapy in EGFR-Mutant Locally Advanced Non-Small-Cell Lung Cancer: The LOGIK0902/OLCSG0905 Intergroup Study Protocol. Clin Lung Cancer 2016;17:75-9. [Crossref] [PubMed]
- Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol 2008;26:2450-6. [Crossref] [PubMed]
- Lu S, Casarini I, Kato T, et al. LAURA: Osimertinib maintenance following definitive chemoradiation therapy (CRT) in patients (pts) with unresectable stage III epidermal growth factor receptor mutation positive (EGFRm) non-small cell lung cancer (NSCLC). Ann Oncol 2020;31:S1385. [Crossref]
- McClatchy DM, Willers H, Hata AN, et al. Modeling Resistance and Recurrence Patterns of Combined Targeted-Chemoradiotherapy Predicts Benefit of Shorter Induction Period. Cancer Res 2020;80:5121-33. [Crossref] [PubMed]
- Akamatsu H, Harada H, Tokunaga S, et al. A Phase II Study of Gefitinib With Concurrent Thoracic Radiotherapy in Patients With Unresectable, Stage III Non-small-cell Lung Cancer Harboring EGFR Mutations (WJOG6911L). Clin Lung Cancer 2019;20:e25-7. [Crossref] [PubMed]
- Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol 2020;31:1056-64. [Crossref] [PubMed]
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2027-39. [Crossref] [PubMed]
Cite this article as: Remon J, Hendriks LEL. Targeted therapies for unresectable stage III non-small cell lung cancer. Mediastinum 2021;5:22.