Traumatic injury to the great vessels of the chest
Introduction and epidemiology
The first description of an aortic injury is attributed to Andreas Vessallius in 1557, being a blunt aortic injury in a victim of an equine event (1). De Bakey’s group reported the first acute repair of an aortic rupture four centuries later (2). Historically, a penetrating wound to the chest, particularly the mediastinum, amounted frequently to certain death. Thankfully, protocols and management strategies have evolved over time to the current surgical approaches when faced with a patient with a potential mediastinal vascular injury, who has survived long enough to arrive alive at the emergency department or trauma bay.
The overall incidence of vascular injuries of the mediastinum remains unclear as the literature traditionally describes chest trauma either in its entirety; or divides it by mechanism of injury; or by specific vascular structures, and frequently mortuary reported incidence is excluded. Mattox reported about an 18% incidence in their series from 1989 and Pate reported on 93 cases in their review of penetrating injury from 1993, with a 70% survival after repair (3,4). A more recent publication examining blunt vascular injury from the authors’ unit showed an overall incidence of blunt vascular injury of around 6% in total and of this total 32% were thoracic, mainly in the form of aortic injury (5).
The mediastinum should be actively assessed with a high degree of suspicion for a mediastinal vascular injury, based on mechanism, especially in high energy transfers, and anatomical location or trajectory of the injury, as in gunshot, stab wounds and impalements. Rather “Rule in” than “Rule out”.
The assessment and management of these patients has substantially advanced over the past two decades with improvements in diagnosis with multi-detector computer tomographic angiography (CTA), frequently protocol based, as well as the undeniable role for a minimally invasive operative strategy as a first line tool in patient care and the recent developments in endovascular therapy.
Anatomy
The mediastinum extends from the thoracic inlet to the diaphragm vertically and is divided into two main compartments, superior and inferior. The latter is then divided into anterior, middle and posterior and their contents are usually described within these subdivisions (6) (Table 1). The focus of this manuscript is on the superior and posterior mediastinum.
Table 1
| Compartment | Organs | Arteries | Veins | Lymphatics | Nerves |
|---|---|---|---|---|---|
| Superior | Thymus, Trachea, Esophagus | Aortic arch, Brachiocephalic trunk, left common Carotid artery, left Subclavian artery | Superior vena cava, Brachiocephalic veins, arch of the Azygos | Thoracic duct | Vagus bilateral, recurrent laryngeal, cardiac, bilateral phrenic nerves |
| Posterior | Esophagus | Descending thoracic aorta | Azygos and hemiazygos veins | Thoracic duct | Vagus, splanchnic, sympathetic chain |
The mediastinal great vessels are relatively protected from both penetrating and blunt trauma due to the surrounding bony ribcage, clavicles and scapulae, along with the spine, however this does not prevent serious injury that may be rapidly fatal if not suitably addressed. The aorta and pulmonary outflow and inflow vessels constitute the most proximal aspect of these vessels with origin at the cardiac ventricles and atria respectively. The superior vena cava (SVC) is formed by the confluence of the two brachiocephalic veins (BCV) and this usually lies just anterior to the aortic arch and the confluence is mostly somewhat to the right of the midline. The Azygous vein enters the SVC from posterior near the right atrium on the right. The hemi-azygous vein forms on the left and anastomoses with the Azygous vein across the spinal column. The inferior vena cava (IVC) ascends via the diaphragm to the right of the midline, ending in a “T-junction” confluence with the SVC flowing into the right atrium. After blood flows through the right atrium and ventricle the pulmonary artery and its trunks dividing left and right curl around the inferior and posterior aspect of the aortic arch to supply the deoxygenated blood to the lungs, returning from the lungs as the lower-pressure pulmonary veins ending in the left atrium. Blood flows through the left atrium and ventricle and exits via the aortic arch into the three (sometimes two) major branches, the brachiocephalic artery (previously called “innominate”), the left common carotid and the somewhat more left posterolateral left subclavian. The former two vessels may have a common origin, sometimes called the “bovine” trunk, with an incidence reported in the population to be around 25–30% (7). Other variants include an absent brachiocephalic trunk with the right common carotid and subclavian arteries branching directly from aortic arch.
Diagnostic approaches
The approach to suspected vascular injury of the mediastinal great vessels will depend on mechanism of injury (blunt versus penetrating) and hemodynamic status. In the case of penetrating injury there is always the added benefit of the entry or exit wounds that guide the probable tract location, while with blunt injury there must be an even higher level of suspicion, especially in case of high-energy acceleration-deceleration type injury.
For penetrating injury, it is important to include the junctional zone of the cervico-mediastinal region since Zone 1 neck penetrating injuries can easily affect the intra-thoracic vasculature (8). The potential for an associated aerodigestive injury must always be considered. In patients with active uncontrolled hemorrhage, it is necessary to proceed directly to operative intervention, while with patients with potential injury and either “soft” or “hard” signs, with apparent controlled bleeding or stability, one would ensure hemodynamic normality and proceed to imaging. For actively bleeding patients attempts at digital or Foley catheter control should be attempted initially (9).
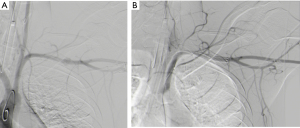
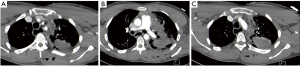
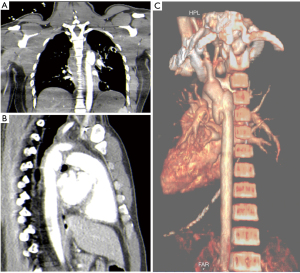
Options for imaging include extended Focused Assessment with Sonar in Trauma (eFAST) to exclude hemothorax, pneumothorax or cardiac tamponade, or abdominal injury, performed in the resuscitation bay, followed by CTA and either contrast swallow or endoscopy to evaluate the esophagus, trachea and main bronchi, especially if surgical emphysema is noted on the CTA (10,11). Another alternative is formal catheter directed angiography (CDA) if retained foreign bodies will increase the “scatter” and prevent adequate diagnostic certainty, commonly the case with retained bullets knife-blades as demonstrated in Figure 1A,B,C,D. CTA is demonstrated in Figure 2 for a case of trans-mediastinal injury.
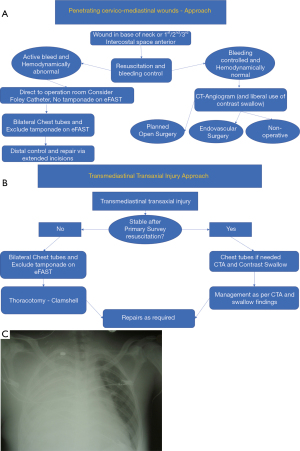
The potential injuries include vascular occlusions from intimal flaps, pseudoaneurysms and arterio-venous fistulae. Active bleeding would result from uncontained arterial and major venous lacerations and transections. Trans-axial penetrating injuries have a slightly different approach (see Figure 3A,B for an approach to these patients). Figure 3Cshows a typical chest X-ray picture of massive right haemothorax as a presenting sign in a case of a chest gunshot wound.
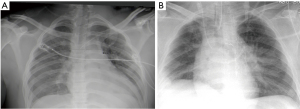
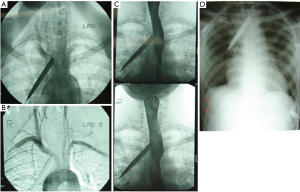
Blunt injury, on the other hand, may cause occult injury and the role of imaging is far more pertinent. On the basic trauma chest-film a widened mediastinum is the main clinical clue (see Figure 4A and 4B showing a widened mediastinum), suggestive of hematoma in the mediastinum and should prompt further imaging.
Pleural capping may suggest aortic or cervical main branch injury. An ipsilateral major hemothorax or esophageal/tracheal displacement further raises concern for a mediastinal vascular injury. Again, the determinant of action is the clinical picture.
If there is a massive hemothorax then urgent ipsilateral anterior thoracotomy is preferred over imaging. For most injuries, however, the cause of any hemodynamic abnormality would be in another cavity, so an eFAST should be performed if the skill exists to direct the surgeon to the correct body cavity, most-often the abdomen.
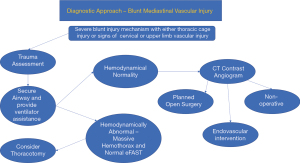
In most cases, however, the patient is hemodynamically almost normal, or responds to the basic fluid resuscitation regimen, thus a high index of suspicion is on the basis of either the mechanism of injury or chest-film findings. Since the likelihood of metal foreign body related scatter is small with blunt trauma, proceeding to CTA is the investigation of choice, considering that associated digestive injury is extremely rare and major airway injury is usually clinically apparent. The common injuries to exclude are blunt aortic rupture, carotid or subclavian intimal injury and pseudoaneurysm, or major venous injury (uncommon) (11-13). Traumatic arterio-venous fistula and pulmonary vessel injuries are exceedingly rare (see Figure 5 for an approach to these patients).
Aortic injury
Blunt thoracic aortic injury (BTAI)
Thoracic aortic injuries are life-threatening surgical emergencies. Blunt and penetrating thoracic aortic injuries differ in incidence from country to country however, motor vehicle collisions account for the majority of blunt injuries. Only 10–20% of patients with BTAI present alive at hospital and the spectrum can be from asymptomatic, to dramatically shocked due to an isolated, contained aortic rupture or, polytraumatised with multiple injuries causing haemorrhagic shock. BTAIs are the second most common cause of early trauma-related deaths following traumatic brain injuries and this accounts for approximately 1.5% of all thoracic trauma (14). The incidence also increases with age and it is rare in pediatric trauma.
The most common anatomic site of injury or tear in BTAI occurs at the aortic isthmus, on the medial luminal aspect of the descending thoracic aorta, distal to the origin of the left subclavian artery (See Figure 6). This is where the relatively mobile aortic arch transitions to the relatively fixed descending aorta (14). Essentially the injury can occur anywhere from the ascending aorta to the bifurcation to the common iliac arteries. Many theories are espoused as to the mechanism of injury at this point (15,16). These include: aortic stretch, intravascular pressure, Water-hammer effect and osseous pinch (Figure 7).
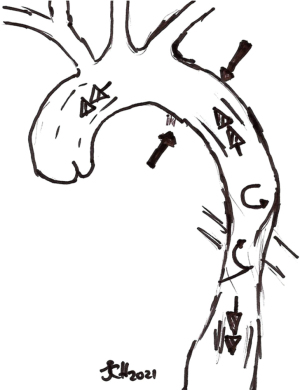
The multivariate approach of a combination of shearing, torsion and stretching with additional hydrostatic forces occurring during sudden deceleration is the most likely manner in which this injury occurs. In addition, tensile strength is weakest at the aortic isthmus resulting in tears of various depths through the layers of the aortic wall, commonly at this point (14). Aortic injuries are graded in terms of the layers of the wall involved coupled with luminal compromise or disruption (Table 2), and the grades lend themselves to guiding management, ranging from conservative in Grade 1 to surgery/endovascular for Grades 2 to 4 (if Grade 4 arrives alive at hospital) (17).
Table 2
| MDCTA-guided grading of thoracic aortic injury | Description of injury | Management principles |
|---|---|---|
| I | Intimal tear | Conservative |
| II | Intramural haematoma | Medical therapy/surgical repair early or delayed |
| III | Pseudoaneurysm | Surgical repair: TEVAR or open repair, early or delayed |
| IV | Rupture | Open emergency repair |
BTAI, blunt thoracic aortic injury; Medical therapy, monitoring in high care or intensive care setting, analgo-sedation, intravenous anti-hypertensive treatment, weight-adjusted prophylactic anticoagulation. TEVAR, thoracic endovascular aortic repair.
Grade 2 appears to be a “grey” zone however, as more patients are now safely being managed by medical treatment alone compared to the previous traditional operative route for both Grades 2 and 3. The mural compromise from intimal tear, to blood filling the dissection plane between intima and media, then compromising and weakening the adventitia to pseudoaneurysm formation and/ or free rupture, is dependent on expeditious initiation of medical therapies to control blood pressure, for control of cardiac afterload, judicious intravenous fluid administration, treatment of coagulopathy if present, and blood and component therapy. Timing of this evolution may occur over a variable period and is dependent on strict adherence to the aforementioned management principles.
The mechanism of injury on history from the patient or from the prehospital transfer team, and the presenting clinical features, as subtle as chest wall bruising, should alert the clinician to the possibility of a thoracic vascular injury. The most common cause of BTAI is rapid deceleration which occurs during motor vehicle collisions, resulting in multi-directional vector forces causing the evolution of the injury, followed by falls from height (14).
Maintaining an astute sense of suspicion is critical. Initial assessment and management in the trauma bay follow the stepwise approach of dealing with immediately life-threatening injuries first as per the Advanced Trauma Life Support (ATLS) program (14,18). If haemodynamically unstable from other life-threatening injuries, the patient should be transferred to the operation room, for operative control of ongoing bleeding and or contamination, and imaging must be deferred. Bedside chest radiography may not be objective in diagnosing a mediastinal vascular injury when the patient is supine, as is frequently the case in the polytraumatized patient. In a seated or semi-recumbent position, the chest radiograph may reveal the typical features of a mediastinal vascular injury (widened mediastinum, apical capping, right tracheal/bronchial deviation etc.) (14,16,19). Once the patient is more stable, protocol driven CTA should be performed. It is theorised that more BTAI are diagnosed in the past 5 to 10 years due on such protocols, with the increasing availability of CTA, and technological advancements in image quality, which may have previously missed grade 1 BTAI, and the increased urgency to actively exclude BTAI when clinically appropriate.
The American Association for the Surgery of Trauma (AAST) grades thoracic vascular injuries on its Organ Injury Scoring scale as I to VI, with grade IV for descending thoracic aorta, Grade V the ascending thoracic aorta and arch, and grade VI being an uncontained total transection of the thoracic aorta which is frequently a post-mortem discovery. Importantly, if there are multiple grade III or IV injuries which are more than 50% of the circumference then these are upgraded by one, and if a grade IV injury is less than 25% of the circumference, it is downgraded similarly (20). CTA may reveal aortic dissection alone which may be classified according to its anatomic location using the De Bakey or Stanford classifications, as for non-traumatic aortic dissection. Management principles are the same.
Transesophageal echocardiography, albeit operator dependent and dependent on equipment availability, is another diagnostic tool that can be used in the trauma resuscitation bay or in the operation room while other causes of haemorrhagic shock are being addressed.
The Abbreviated Injury Score (AIS) attributes a value of 4 to Grades 1 and 2 of BTAI, and a 5 to grades 3 and 4. This translates to Injury Severity Scores of 16 and 25 respectively, thus further defining the gross severity of this specific injury which has further implications on outcomes (20).
An appropriate timing and treatment strategy is required in the face of multiple, often complex, injuries. In this scenario, it is prudent to address other life-threatening or potentially life-threatening injuries first, continue with resuscitation as required and once the patient is deemed stable, then CTA can be undertaken and operative invention of the BTAI either more than 24 hours later, or delayed for a few days. In the case of isolated BTAI however, early surgical intervention (<24 hours) after efficacious resuscitation, is acceptable. Once this diagnosis has been made, intravenous access should be obtained without unnecessary volume expansion, and strict adherence to intravenous blood pressure control should be initiated to facilitate “permissive hypotension” while maintaining vital organ perfusion. The risk of rupture has been shown to decrease from 12% to 1.5% with effective anti-impulse therapy (14,17). Esmolol is the traditional drug of choice, however locally, we use a titrated labetalol infusion first, due to unavailability of Esmolol as an intravenous infusion. The target is a systolic blood pressure around 100 mmHg, or means less than 80 mmHg, with a pulse rate around 100/min. Other drugs which may be used in combination with beta blockade, or as an alternative, are diltiazem, nitroprusside or nitroglycerin, all with vigilant blood-pressure monitoring in a high care ward or intensive care unit (14). It is imperative to remember to commence the beta blockade prior to any of the other drugs mentioned above to prevent acute shear- stress on the weakened aortic wall due to a reflex tachycardia.
Management
Thoracic endovascular aortic repair (TEVAR) has superseded open repair over the past two and half decades and has become the therapeutic option of choice for TAI, especially those from blunt trauma, however there are reports of addressing penetrating TAI successfully via this route as well (19,21). Many studies have described the advantages of TEVAR over the less preferred, open repair yet there are no supporting large, randomised control trials (22). Importantly, the delayed route of repair allows for more optimal management of other injuries and improvement in haemodynamic status.
Hybrid operating rooms are advantageous as the surgical team and the patient are prepared for both endovascular and open procedures and may provide the ideal environment to combine endovascular therapies as bridging treatments to operative or open surgical intervention. Multi-disciplinary teams can assess and engage for the benefit of the patient. Extracorporeal membrane oxygenation (ECMO) has been described for severe respiratory distress in trauma as well as in the intraoperative management of BTAI repair and hybrid operating rooms may be able to offer this to patients in extremis (21).
Grade I TAI is managed conservatively, using anticoagulants, with planned repeat CTA imaging (23). Grade II TAI have recently been shown to have good outcomes when managed nonoperatively, however this is not yet described in consensus guidelines (21). In addition, studies over the past few years have described the conservative management of small pseudoaneurysms, whereas the Society for Vascular Surgery document on Endovascular repair of traumatic thoracic aortic injury: Clinical practice guidelines of the Society for Vascular Surgery, recommends that Grades II onwards require endoluminal stenting or repair (21,23). Follow-up imaging and medical therapy is addressed in local guidelines (23). Figure 8A and 8B demonstrate the angiogram images before and after TEVAR in a recent case at this facility.
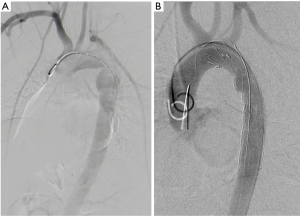
Questions around long term surveillance of patients who have undergone TEVAR, specifically looking for complications, endoleaks, the durability of endografts, optimal timing of intervention when other injuries prevail, remain.
The advantages of TEVAR include lower mortality (9%), no need for cardiac bypass, less risk to the spinal cord from ischemia, no need to change patient position intra-operatively, shorter operating and hospital length of stay, reduced affects on associated injuries, and reduced blood loss (19,21,23). The disadvantages are the endovascular approach include the need for expertise in performing the procedure, vessel size for access, lumen size for the graft (affected by hemodynamic status) and landing-site availability (2cm proximal and 10 cm distal), however crossing the left subclavian appears safe when needed (19,21,24,25). Anatomic variants may be a factor, however. There is a theoretical risk of acute kidney injury from contrast (although this is highly disputed) (26,27). The TEVAR is not good for addressing more proximal arch injury but may be used as part of a hybrid approach.
On the other hand, open repair is possible for the general or thoracic surgeon without endovascular training, allows rapid access to the injury in patients in extremis, but carries higher risks of spinal ischaemia (around 11%) and acute kidney injury due to blood loss. Polytrauma patients, especially those with lung contusion may do worse with early or open surgery. The mortality rate is up to 16% with open surgery, however it remains the option of choice for aortic root and arch injury (19,21,23).
Penetrating thoracic aortic injury (PTAI):
PTAI are less common than BTAI and are most frequently due to gunshots and stabs. There are also descriptions in the literature of foreign bodies impaled into or lodged within the mediastinum (28). PTAI are lethal injuries and many patients present in extremis due to rapid exsanguination prior to arrival at the trauma bay. Assessment follows as described for BTAI however, CTA is only considered if the patient remains hemodynamically stable and if not, then they are swiftly taken to the operating room for open surgery, i.e. thoracotomy or sternotomy. CTA in the stable patient with PTAI may be challenging due to frequent scatter from in-situ foreign bodies. Such patients should undergo CDA either in lieu of CTA or in addition to CTA if they remain stable. CDA allows for an endovascular solution for these patients in diagnosing the specific vascular structure injured, its pathology, the role of the foreign body in blood vessel tamponade if present, intravascular presence of the foreign body and provides the means to treat the injury via stenting or endograft (28). Pathological types may be similar to BTAI but may be more complex including aorto-caval or arterio-venous fistulas. Hybrid trauma operating theatres may provide the ideal environment to combine endovascular therapies as bridging treatments to operative open surgical intervention, e.g., REBOA or endograft placement.
Aortic branch injury
The aortic branches that may be injured, mainly through penetrating injury, to a lesser extent through blunt injury are the brachiocephalic, the left common carotid and the left subclavian in the retrosternal space, prior to entering the neck (8). These injuries carry a high mortality in the pre-hospital environment and a moderate mortality once in hospital (29,30). The spectrum of injury is from “minimal” injury with intimal damage, various degrees of laceration (with or without pseudoaneurysm) and arterio-venous fistula (Figure 9). The classification of the blunt injuries, as described by Biffl and colleagues, is outlined in Table 3 (31). Management depends on the injury severity with antithrombotic therapy for 7–10 days for grades 1 and 2, stent or surgical repair of grade 3 and 4 unless a dense neurological fallout is already present and grade 5 is treated with either open surgery or a stent could be considered. Antiplatelet therapy for a further 3 months and then re-imaging is advised (11,12,32).
Table 3
| Grade | Description |
|---|---|
| Grade 1 | Intimal injury without occlusion <25% of circumference narrowing |
| Grade 2 | Partial laceration or dissection >25% of circumference narrowing; Intramural hematoma |
| Grade 3 | Pseudoaneurysm |
| Grade 4 | Vessel occlusion |
| Grade 5 | Transection |
The modern management approaches to these injuries have undergone a major shift over the recent past with a move toward endovascular management where the skill exists, in particular for blunt injury or contained penetrating injury (30,32-35). For open surgery these vessels require sternotomy for proximal control in most cases, although the 3rd space anterior mini-thoracotomy may be an option for proximal control of the left subclavian (see Figure 10).
Traditionally anterolateral thoracotomy is a poor access to these vessels in most cases and the “trap-door” approach should be regarded as obsolete due to the poor functional outcome thereof.
Operative technique—the author’s approach
The patient should be cleaned and draped such that the entire chest, upper abdomen and the neck and shoulders are accessible. Ideally “free-draping” of the arms is helpful for subclavian access. A prophylactic dose of either 2 gKefazolin or 2.4 g Amoxicillin-clavulanic acid (or equivalent) is administered prior to incision.
Once the sternum is open, if there is no active bleeding, one proceeds to dissect the substernal soft tissue, then encircling and either elevating or dividing the SVC proximal to the confluence of the two BCV’s and thus gain access to the aortic root and arch. After looping the various branch vessels using a Heiss or Mixter dissector proximal control can be attained. Proximal control with non-crushing vascular clamps (small Satinski, Dadek, Glover or Leland-Jones type) should reduce bleeding risk, but distal back-bleeding from the vessels is common. Distal control depends on the likely involved vessel, namely from front to back on the arch as follows, slinging the vessel with loops as soon as possible:
- Brachiocephalic artery—subclavian access via a supra-clavicular extension (protecting the phrenic nerve prior to dividing the Scalenus muscle) or right Common Carotid via an anterior sternomastoid extension;
- Left Common Carotid artery—left anterior sternomastoid incision;
- Left subclavian—left supraclavicular incision, protecting the phrenic nerve prior to dividing the Scalenus muscle;
- It is advisable to also get vascular slings around the accompanying large veins.
Once proximal and distal control is attained, heparin IV bolus is given and then the vascular injury can be repaired using the options are as follows:
- Primary lateral repair after debridement and embolectomy catheters up/down;
- Primary end-to-end repair after debridement and embolectomy catheters up/down;
- Debridement, embolectomy and lateral venous or synthetic patching;
- Resection of damaged segment, embolectomies and then venous interposition or synthetic graft reconstruction;
- Severe injuries of the subclavian vessels in a damage control setting can be ligated and there is a risk of limb-loss;
- Carotid ligation is inadvisable, unless there is already dense neurology in an unstable patient, due to the risk (albeit small) of hemorrhagic infarct transformation (35).
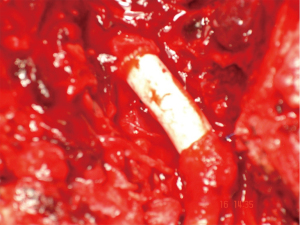
For synthetic graft patch or interposition, it is advisable to use PTFE grafts and not Dacron due to the reported higher sepsis rates with the latter, supposedly due to the porous material in a contaminated field (36). This is important with the BCA and the proximal CCA as these are fairly friable vessels and should not be repaired under any tension, so interposition venous or synthetic grafts are recommended (see Figure 11—an example of a synthetic PTFE graft of the BCA). Suture material is either PTFE or monofilament polypropylene, avoiding any absorbable material.
Closure of the platysma and subcutaneous tissues along with skin, followed by standard closure of the sternotomy is the next step. Drains are left for lymphatic leaks in the soft tissue of the neck (closed suction type) and standard post-sternotomy drains in the sub-sternal space (and pericardial space if this was opened).
Endovascular option
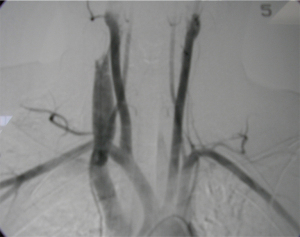
The alternative strategy, pioneered by du Toit and colleagues from Tygerberg/University of Stellenbosch in Cape Town, South Africa, is the use of covered stents placed via endovascular access (32-35). Their experience and longer-term follow-up have proved that this is a feasible management option for patients who are both hemodynamically normal, provided the guidewire is passible beyond the injury to ensure safe stent placement under fluoroscopic guidance (32-35). These stents are ideal in the proximal carotid and first-part subclavian, thus avoiding the need for sternotomy access. Figure 12A,B show recent stable left subclavian injuries managed by the local team before and after stent placement.
Major venous injury
Injuries of the SVC and IVC (TVIs) are described less frequently, compared with arterial injuries within the same compartment. This is likely due to a common association of TVIs with other vascular or organ injuries and these injuries are frequently non-survivable (13).
The mechanism of injury to the superior vena, brachiocephalic veins and suprahepatic inferior vena is the same as for thoracic aortic injuries however, penetrating venous injuries occur much more commonly than blunt (37) (see Figure 13—AV fistula between Left CCA and BCV). Gunshots and stabs are the most common causes of penetrating trauma however, many iatrogenic injuries are being documented in the literature, for example, following central venous catheter placement for multiple intravenous ports or inotrope administration, dialysis catheter insertion and balloon angioplasty for central venous thrombosis (13,37,38).
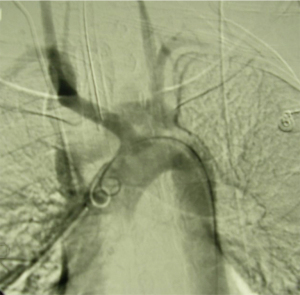
Blunt TVI, although uncommon, can be encountered following motor vehicle collisions where the mechanism of the TVI is likely due to the vectors applied to the vessel during rapid acceleration-deceleration, as in BTAI, where the SVC is relatively mobile in comparison to the atria, or due to significant compression of the vessel between the sternum and vertebral column (39). An avulsion injury of the SVC from the right atrium has been described in the literature, having presented with cardiac tamponade due to hemopericardium since the SVC had ruptured intrapericardially (39,40). The confluence of the SVC with the right atrium is a likely site of major venous injury (40).
Injuries to the suprahepatic and retrohepatic portion of the IVC carry the highest mortality but fortunately most IVC injury is infra-renal (41). Blunt IVC injury has a higher mortality rate than that caused by penetrating injury (41). Whether penetrating or blunt SVC, BCV or intrathoracic IVC injuries are encountered, the patient is usually in hypovolaemic shock. The insertion of intercostal drains may reveal massive hemothoraces which necessitate resuscitative thoracotomy or sternotomy, especially in penetrating TVI. Identifying this patient in extremis is pivotal in order to preserve life. The operative options include lateral venorrhaphy, intracaval shunt, PTFE-patch and endovascular stenting in the more stable patient, and ligation as swiftly as possible for those in extremis (42).
The injured suprahepatic IVC is extremely challenging to manage operatively and requires cardiopulmonary bypass in most centres (43,44). Blunt injury may occur as an avulsion injury at the atrio-caval junction and may be self-contained or tamponaded by surrounding structures. Management depends on overall patient stability and more recently, endovascular management is being performed. provides a lifeline as these patients would have succumbed to massive intraoperative blood loss. Options for treatment include conservative, endovascular (even if pseudoaneurysm exists), ligation or packing and definitive vascular repair (43).
Iatrogenic SVC injuries occur mainly at the confluences of the brachiocephalic veins and SVC and occur with equal frequency whether the left or right subclavian or internal jugular veins are cannulated. The tip of the hard-plastic dilator is usually the culprit tearing through the wall of the SVC, BCV or its confluence, resulting in cardiac tamponade or a massive haemothorax, both of which require resuscitative thoracotomy to preserve life (13). The literature also describes intrapleural CVC placement which requires fluoroscopic and angiographic guidance for removal usually by a vascular surgeon. The SVC can rupture during elective venoplasty and stenting for other conditions such as SVC thrombosis from long-term indwelling catheters used for dialysis or parenteral nutrition (13). The tip of the catheter may also erode through the venous wall over time resulting in perforation of a large vessel with dramatic consequences (13).
Blunt brachiocephalic injury is uncommon but it may occur during high speed motor vehicle crashes, sometimes associated with bony distraction of the vessels by sternoclavicular joint disruption (45), rib and clavicular fractures. On imaging, these injuries would appear as a contained perivascular or mediastinal haematoma (13). Penetrating BCV injuries are more common than blunt injuries and one should be vigilant when assessing a patient with a penetrating cervicomediastinal wound. CTA of the aortic arch and great vessels is indicated and may be combined with CDA for further diagnostic or therapeutic purposes. Active contrast extravasation and an expanding mediastinal haematoma warrants emergent operative intervention (13). For the patient in extremis, ligation of all major thoracic venous injuries can be performed without the risk of chronic oedema as this has been shown to be transient and spontaneously resolve (42).
Less common arterial or venous injuries
Pulmonary artery
Injury to the main intrathoracic great vessels remain uncommon. Major transection of either the pulmonary artery (PA) or veins (PV) are often rapidly fatal, thus explaining the limited clinical data on these injuries. The PA is a low-pressure system and thus the likely presenting pathology is a large hemothorax, or a finding of a suspected pseudoaneurysm on CTA. Access to these vessels may require either sternotomy or postero-lateral thoracotomy and usually requires bypass facilities, which are often not readily at hand in the unsavoury hours where trauma cases present, especially when actively bleeding (46,47). Abbas, in his recent commentary, makes the following points about management: direct pressure will control most bleeding; small pseudoaneurysms are often managed non-operative means, or with coil-embolization via fluoroscopy. He emphasizes the need for cardiac bypass for proximal injury (46). The proximal injury can easily rupture into the pericardial sac and would be a rare cause of tamponade, thus treated by thoracotomy and repair, albeit difficult with the beating heart. For more distal injury balloon-catheter or hilar clamping may assist in hemorrhage control. Pneumonectomy may be the only option in some cases.
Pulmonary vein
These injuries are most commonly rapidly fatal and present with a rapid onset large hemothorax that may briefly tamponade the injury given the low-pressure system involved. Massive tears and intra-pericardial injury leading to tamponade seldom reach hospital (13). Access to the injury is best achieved through an ipsilateral thoracotomy and with clamshell extension if needed. Temporary hilar clamping may assist vascular control. Direct repair using continuous polypropolene suture is the treatment of choice for proximal injury or ligation for branch injury is advised. Proximal injury can be approached via sternotomy and dissection between the SVC and Aorta (48).
Azygous and hemi-azygous injury
The azygous vein is seldom injured by either penetrating or blunt trauma and may present as a widened mediastinum or large right-sided hemothorax (13,49-51). Most of the blunt injuries are associated with motor vehicle crashes, often with mid-thoracic vertebral injury. Less than 50 caseshave been reported from either cause since the initial reports by Wall and colleagues in 2006 where they reviewed 22 cases from penetrating injury over 40 years. There was a clear predominance of gunshot wounds and they described a mortality of 36% (49). A case report in 2010 added a single blunt mechanism case to the report of 20 cases of blunt injury originally recorded in 2006 (50). Actively bleeding venous injury is best approached via a right anterolateral or posterolateral thoracotomy and treatment is venous ligation. A similar approach on the left would apply to the hemi-azygous, however this is even less likely to cause severe bleeding and is mostly identified on CTA performed for a widened mediastinum on plain chest film. Haemodynamically normal patients can potentially be managed non-operatively (51).
Conclusions
Mediastinal great vessel injury carries a high mortality and morbidity, with motor-vehicle trauma and gunshot wounds the primary causes of such injury. The management of Aortic injury has taken a major turn toward endovascular management with reduced morbidity, while the other main branch injuries are mainly associated with penetrating trauma. Even here endovascular options are increasingly being used. Less common injuries to other mediastinal vessels occur and these have less supporting data on how management should proceed.
Take-home messages
- Blunt aortic injury is more readily diagnosed early due to the increased use of “pan-scan” of whole-body trauma CTA in the early phase of care, however early control of blood pressure and timely aortic endograft management have become the mainstay of treatment.
- Penetrating trauma to the aorta and its main branches carry high early mortality, however early surgical intervention and vascular control have reduced the mortality over time.
- Blunt carotid and subclavian injury should be actively searched for via CTA in high-risk patient groups.
- Major venous injury is usually found on CTA or at thoracotomy/sternotomy for active bleeding and can be addressed by suture-repair or most often ligation. Simple repairs should be attempted, most others ligated with the exception of the venae cavae.
- Bypass is seldom immediately available and mostly not required for these injuries with the exception of very proximal injury.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Simon R. Turner) for the series “Traumatic Injuries of the Mediastinum” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at: https://dx.doi.org/10.21037/med-21-15). The series “Traumatic Injuries of the Mediastinum” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Di Marco L, Pacini D, Di Bartolomeo R. Acute Traumatic Thoracic Aortic Injury: Considerations and Reflections on the Endovascular Aneurysm Repair. Aorta (Stamford) 2013;1:117-22. [Crossref] [PubMed]
- Demetriades D. Aortic Injuries: Crossing the rubicon. J Am Coll Surg 2012;214:247-59. [Crossref] [PubMed]
- Mattox KL, Feliciano DV, Burch J, et al. Five thousand seven hundred sixty cardiovascular injuries in 4459 patients. Epidemiologic evolution 1958 to 1987. Ann Surg 1989;209:698. [Crossref] [PubMed]
- Pate JW, Cole FH Jr, Walker WA, et al. Penetrating injuries of the aortic arch and its branches. Ann Thorac Surg 1993;55:586. [Crossref] [PubMed]
- Muckart DJJ, Pillay B, Hardcastle TC, et al. Vascular injuries following blunt polytrauma. Eur J Trauma Emerg Surg 2014;40:315-22. [Crossref] [PubMed]
- Rizvi S, Wehrle CJ, Law MA. Anatomy, Thorax, Mediastinum Superior and Great Vessels. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2020.
- Ahn SS, Chen SW, Miller TJ, et al. What Is the True Incidence of Anomalous Bovine Left Common Carotid Artery Configuration? Ann Vasc Surg 2014;28:381-5. [Crossref] [PubMed]
- Islam J, Laing GL, Bruce JL, et al. Outcomes for cervicomediastinal vascular trauma managed by a vascular subspecialist led vascular trauma service. S Afr J Surg 2016;54:15-9. [PubMed]
- Scriba M, MacPherson D, Edu S, et al. An Update on Foley Catheter Balloon Tamponade for Penetrating Neck Injuries. World J Surg 2020;44:2647-55. [Crossref] [PubMed]
- Nel L, Whitfield Jones L, Hardcastle TC. Imaging The Oesophagus After Penetrating Cervical Trauma Using Water Soluble Contrast Alone: Simple, Cost Effective And Accurate. Emerg Med J 2009;26:106-8. [Crossref] [PubMed]
- Hundersmarck D, Sloof WM, Homans JF, et al. Blunt cerebrovascular injury: incidence and long-term follow-up. Eur J Trauma Emerg Surg 2021;47:161-70. [Crossref] [PubMed]
- Cothren CC, Moore E, Biffl W, et al. Anticoagulation is the gold standard therapy for blunt carotid injuries to reduce stroke rate. Arch Surg 2004;139:540-5. [Crossref] [PubMed]
- Haq AA, Restrepo CS, Lamus D, et al. Thoracic venous injuries: an imaging and management overview. Emerg Radiol 2016;23:291-301. [Crossref] [PubMed]
- Akhmerov A, DuBose J, Azizzadeh A. Blunt Thoracic Aortic Injury: Current Therapies, Outcomes, and Challenges. Ann Vasc Dis 2019;12:1-5. [Crossref] [PubMed]
- Richens D, Field M, Neale M, et al. The Mechanism of injury in blunt traumatic rupture of the aorta. Eur J Cardiothorac Surg 2002;21:288-93. [Crossref] [PubMed]
- Mouawad NJ, Paulisin J, Hofmeister S, et al. Blunt thoracic aortic injury - concepts and management. J Cardiothorac Surg 2020;15:62. [Crossref] [PubMed]
- Azizzadeh A, Keyhani K, Miller CC, et al. Blunt traumatic aortic injury: Initial experience with endovascular repair. J Vasc Surg 2009;49:1403-8. [Crossref] [PubMed]
- Committee on Trauma, American College of Surgeons. ATLS Advanced Trauma Life Support 10th Edition Student Course Manual. Chicago IL, American College of Surgeons; 2019.
- Scalea TM, Feliciano DV, DuBose JJ, et al. Blunt Thoracic Aortic Injury: Endovascular Repair Is Now the Standard. J Am Coll Surg 2019;228:605-10. [Crossref] [PubMed]
- Appendix B18. IN: Boffard KD. Manual of Definitive Surgical Trauma Care. 5th Ed. Boca Raton, FL: Taylor and Francis, 2019:369.
- Lee WA, Matsumura JS, Mitchell RS, et al. Endovascular repair of traumatic thoracic aortic injury: Clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg 2011;53:187-92. [Crossref] [PubMed]
- Pang D, Hildebrand D, Bachoo P. Thoracic endovascular repair (TEVAR) versus open surgery for blunt traumatic thoracic aortic injury. Cochrane Database Syst Rev 2019;2:CD006642 [Crossref] [PubMed]
- Naidoo D. Section D: Thoracic aortic trauma: Who and how to treat. IN: Vellor M (Editor). Thoracic Aortic Interventions. Vascular Society of South Africa Guidelines 2019. Accessed on 05/01/2021. Available online: http://www.vascularsociety.co.za/wp-content/uploads/2015/08/Thoracic-aortic-interventions-2012.pdf
- Demetriades D, Velmahos GC, Scalea TM, et al. Operative repair or endovascular stent graft in blunt traumatic thoracic aortic injuries: results of an American Association for the Surgery of Trauma multicenter study. J Trauma 2008;64:561-70; discussion 570-1. [Crossref] [PubMed]
- Jonker FHW, Verhagen HJM, Mojibian H, et al. Aortic endograft sizing in trauma patients with hemodynamic instability. J Vasc Surg 2010;52:39-44. [Crossref] [PubMed]
- Davenport MS, Parazella MA, Yee J, et al. Use of Intravenous Iodinated Contrast Media in Patients with Kidney Disease: Consensus Statements from the American College of Radiology and the National Kidney Foundation. Radiology 2020;294:660-8. [Crossref] [PubMed]
- Hinson JS, Ehmann MR, Fine DM, et al. Risk of Acute Kidney Injury After Intravenous Contrast Media Administration. Ann Emerg Med 2017;69:577-86.e4. [Crossref] [PubMed]
- Omran N, Habal P, Mandak J, et al. Penetrating Aortic Injury. Ann Thorac Surg 2014;97:e119 [Crossref] [PubMed]
- McKinley AG, Carrim AT, Robbs JV. Management of proximal axillary and subclavian artery injuries. Br J Surg 2000;87:79-85. [Crossref] [PubMed]
- du Toit DF, Odendaal W, Lambrechts A, et al. Surgical and Endovascular Management of Penetrating Innominate Artery Injuries. Eur J Vasc Endovasc Surg 2008;36:56-62. [Crossref] [PubMed]
- Biffl WL, Moore EE, Offner PJ, et al. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma. 1999;47:845-53. [Crossref] [PubMed]
- du Toit DF, Lambrechts AV, Stark H, et al. Long-term results of stent graft treatment of subclavian artery injuries: Management of choice for stable patients? J Vasc Surg 2008;47:739-43. [Crossref] [PubMed]
- du Toit DF, Leith JG, Strauss DC, et al. Endovascular management of traumatic cervicothoracic arteriovenous fistula. Br J Surg 2003;90:1516-21. [Crossref] [PubMed]
- du Toit DF, Strauss DC, Blaszczyk M, et al. Endovascular Treatment of Penetrating Thoracic Outlet Arterial Injuries. Eur J Vasc Endovasc Surg 2000;19:489-95. [Crossref] [PubMed]
- du Toit DF, Van Schalkwyk GD, Wadee SA, et al. Neurologic outcome after penetrating extracranial arterial trauma. J Vasc Surg 2003;38:257-62. [Crossref] [PubMed]
- Feliciano DV, Mattox KL, Graham JM, et al. Five-year experience with PTFE grafts in vascular wounds. J Trauma. 1985;25:71-82. [Crossref] [PubMed]
- Turkyilmaz A, Karapolat S, Kilic M, et al. The Perforation of the Superior Vena Cava Secondary to the Left Subclavian Dialysis Catheter. Vasc Endovascular Surg 2017;51:95-7. [Crossref] [PubMed]
- Liu YW, Lee YL, Hsieh JS, et al. Thoracoscopic purse-string suture technique for managing superior vena caval impalement from central venous catheterization. J Trauma Acute Care Surg 2017;83:899-902. [Crossref] [PubMed]
- Bakaeen FG, Wall MJ, Mattox KL. Successful Repair of an Avulsion of the Superior Vena Cava from the Right Atrium Inflicted by Blunt Trauma. J Trauma 2005;59:1486-8. [Crossref] [PubMed]
- Robbs JV, Reddy E. Management options for penetrating injuries to the great veins of the neck and superior mediastinum. Surg Gynecol Obstet 1987;165:323-6. [PubMed]
- Bouabdallaoui N, Debbagh H, Schoell T, et al. Surgical Management of Undiagnosed Laceration of Superior Vena Cava Caused by Blunt Trauma. Ann Thorac Surg 2016;101:1972-4. [Crossref] [PubMed]
- Nair R, Robbs JV, Muckart DJ. Management of penetrating cervicomediastinal venous trauma. Eur J Vasc Endovasc Surg 2000;19:65-9. [Crossref] [PubMed]
- Leon M, Chavez L O, Chavez A, et al. Blunt Aortic / Inferior Vena Cava Injury: Are We Consistently Providing the Same Level of Care? Cureus 2020;12:e6832 [Crossref] [PubMed]
- Navsaria PH, Chowdhury S, Nicol A, et al. Penetrating Trauma to the Mediastinal Vessels: a Taxing Injury. Curr Trauma Rep 2016;2:1-10. [Crossref]
- di Mento L, Staletti L, Cavanna M, et al. Posterior sternoclavicular joint dislocation with brachiocephalic vein injury: a case report. Injury 2015;46:S8-10. [Crossref] [PubMed]
- Abbas AE. Traumatic injury of the pulmonary artery: Transection, rupture, pseudoaneurysm, or dissection? Sometimes semantics do matter. J Thorac Cardiovasc Surg 2016;152:1437-8. [Crossref] [PubMed]
- Deneuville M. Injury of the pulmonary artery and its branches due to penetrating chest trauma. Ann Vasc Surg 2000;14:463-7. [Crossref] [PubMed]
- Chapter 8, The Chest. In: Boffard KD. editor. Manual of Definitive Surgical Trauma Care, 5th Ed. Boca Raton, FL: Taylor and Francis, 2019:369.
- Wall MJ, Mattox KL, De Bakey M. Injuries to the azygous venous system. J Trauma 2006;60:357-62. [Crossref] [PubMed]
- Endara SA, Davalosa GA, Nunez MF, et al. Azygous vein laceration secondary to blunt thoraco-abdominal Trauma. Interactive CardioVascular and Thoracic Surgery 2010;11:342-4. [Crossref] [PubMed]
- McDermott C, O'Connor G, McGovern E, et al. Conservative management of azygous vein rupture in blunt thoracic trauma. Case Rep Crit Care 2012;2012:147614 [Crossref] [PubMed]
Cite this article as: Naidoo S, Hardcastle TC. Traumatic injury to the great vessels of the chest. Mediastinum 2021;5:26.