Giant tumors of the posterior mediastinum: a narrative review of surgical treatment
Introduction
The term mediastinum is used to define thoracic compartments, except for the lungs. The mediastinum is mainly divided into three compartments: anterior, middle, and posterior. However, some authors prefer the “paravertebral sulcus” instead of the posterior mediastinum (1). Tumors arising from structures located in the paravertebral sulcus (e.g., neurogenic tumors) are considered posterior mediastinal tumors. The sympathetic chain, proximal intercostal nerves and vessels, paraesophageal and intercostal lymph nodes, and distal azygos veins are also located in this compartment.
Some authors consider the posterior mediastinum the region between the pericardium and the vertebrae (2). According to the subject definition, the esophagus and descending thoracic aorta are also considered in the posterior mediastinum.
This difference in terminology is due to the close anatomic relationship between the mentioned anatomical structures. Therefore, all three compartments are almost always affected by “giant posterior mediastinal tumors”. Thus, in one article, a lesion described as a middle mediastinal pathology may be described as a posterior mediastinum in another.
This review aims to identify the term “giant mediastinal tumor” and the etiology, clinical features, diagnostic methods, pathological types, surgical methods applied, and technical details of these methods for the treatment of these tumors. We present the following article in accordance with the Narrative Review reporting checklist (available at https://med.amegroups.com/article/view/10.21037/med-21-39/rc).
Histopathological subtypes
The masses located in the posterior mediastinum are in a wide range of diseases. Neurogenic tumors (e.g., schwannoma, ganglioneuroma, and neuroblastoma) constitute almost 80% of posterior mediastinal tumors (3). The age of onset may vary based on the histopathological subtype. Tumors originating from the sympathetic ganglia are primarily observed in childhood, whereas other neurogenic tumors are usually seen in adults. Nearly 90% of adult neurogenic tumors are nerve sheath tumors and are asymptomatic unless they reach a significant size. Tumors, such as pheochromocytoma, which originate from paraganglionic cells of the sympathetic nervous system, are hormonally active. Thus, careful presurgical preparation is required. Intrathoracic lymph node hyperplasia (Castleman disease), soft tissue tumors (mesenchymoma), sarcomas, and intrathoracic goiter could also be settled in the posterior mediastinum when they significantly increase in size (4-8). On the other hand, giant esophageal tumors [gastrointestinal stromal tumors (GIST), leiomyoma] may invade the posterior mediastinum (9,10).
Methods
We reviewed the literature to identify previous studies on the surgical treatment of giant posterior mediastinal tumors. A search was conducted on the PubMed database with “posterior mediastinum, giant mass” or “posterior mediastinum, tumor, giant” keywords. The search was limited to English language and full-text available studies published between 1984–2021 (Table 1). The generally accepted description of the term “giant” referring to “10 cm and above in size” for other intrathoracic lesions was also accepted in this review (11).
Table 1
| Items | Specification |
|---|---|
| Date of search (specified to date, month and year) | 31.10.2021 |
| Databases and other sources searched | PubMed |
| Search terms used (including MeSH and free text search terms and filters) | “posterior mediastinum, giant mass” or “posterior mediastinum, tumor, giant” |
| Timeframe | 1984–2021 |
| Inclusion and exclusion criteria (study type, language restrictions etc.) | Inclusion criteria: all types of articles available in full text in English; articles which include posterior mediastinal mass larger than 10 cm in diameter; case reports underwent resection and included histopathological diagnosis; to define of mediastinal compartments we used related textbooks (1,2) |
| Exclusion criteria: Article was not reached full text | |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | All authors of the study independently from each other performed a PubMed search according to inclusion criteria. Then, the articles were evaluated collectively, and the ones that met the criteria were cited and a review was written |
Evaluation
Mediastinal tumors are usually asymptomatic. However, most patients with giant tumors are admitted to clinics with respiratory system complaints (7,12). Chest pain, cough, chest tightness, and shortness of breath are common symptoms (5,9). Due to compression or invasion of the surrounding tissues, neurogenic symptoms, back pain, or difficulty swallowing can also be observed (7,13). Apically located mediastinal masses may cause superior vena cava syndrome due to the compression (14).
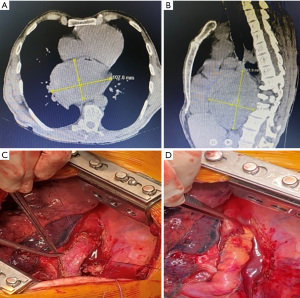
Radiological evaluation begins with a chest roentgenogram. These tumors are mainly observed as radioopacity. Rarely, they may cause pleural effusion (15). The primary diagnostic test is thoracic computed tomography (CT), in which more detailed information about these lesions is revealed (12). If the tumor is close to the spinal cord or spinal cord compression symptoms are available, magnetic resonance imaging (MRI) is required (12). In the case of hemilaminectomy or hemivertebrectomy, an operation is planned with the neurosurgery department. MRI is similarly helpful when there is a suspected invasion of the esophagus or other mediastinal structures (10,16). The relationship between the lesion and the esophagus in patients with swallowing difficulties will be revealed by esophagoscopy or endoscopic ultrasonography. Although giant tumors adjacent to the esophagus can be dissected from the esophagus wall, esophagectomy may be required in cases of severe mucosal damage during dissection (Figure 1). A bronchoscopic evaluation may be considered when a suspected invasion of the carina or trachea is revealed by CT or MRI (7). Although specific findings are not revealed by positron emission tomography (PET), they can also be used to characterize and demonstrate the extent of lesions in giant tumors (10,16). Although histopathological diagnosis is not necessary before surgery for giant posterior mediastinal masses, it could be achieved by radiology-guided biopsy (12,17,18). However, in some cases, the diagnosis cannot be achieved by fine-needle aspiration or even by core biopsy (19). Histopathological diagnosis is not mandatory for a surgical decision.
Surgical treatment
The primary treatment for giant posterior mediastinum tumors is surgical excision. In these patients, a posterolateral thoracotomy is generally preferred. Although it is a painful incision, it is still the most common method in giant tumor treatment (Table 2). The video-thoracoscopic approach, preferred for smaller lesions, is not helpful for giant tumors due to insufficient exposure. Because the posterior mediastinum is a small space, the relationship between the lesion, the esophagus, and the airways should be considered (8,18). However, these structures are compressed but are not invaded by most of these giant tumors (Figure 2). Rarely, a thoracoabdominal approach is required for the surgery of giant tumors (17,18). For tumors invading the lung or chest wall, en bloc resection may rarely be considered. When diaphragm resection is needed, it can be reconstructed with a polytetrafluoroethylene (PTFE) graft (18).
Table 2
| Author(s) | Age (years) | Sex | Location | Size (cm) | Symptom | Approach | Diagnosis |
|---|---|---|---|---|---|---|---|
| Hsu et al. (2005) | 50 | M | Dumble-shape | 14×11×5.5 | Dyspnea, dysphagia, jugular distension | PL Th | Fibrolipoma |
| Luca et al. (2013) | 64 | F | Right PV | 15×10×8 | Dyspnea, fatigue, cough | PL Th | Granular cell tumor |
| Hayat et al. (2011) | 45 | F | Left PV | 21×14×9 | Shortness of breath | PL Th | Ganglioneuroma |
| Bhatti et al. (1984) | 61 | M | Right PV, Sup mediastinum | 8×10 | Chest pain, dyspnea | Th | Castleman disease |
| Chen et al. (2013) | 58 | M | Right PV | 10×9×9 | Chest tightness, dyspnea | PL Th | Thyroid hyperplasia, goiter |
| Elnady et al. (2020) | 17 | F | Left PV | 20×15×8 | Back pain | P Th | Ganglioneuroma |
| Zhao et al. (2016) | 54 | F | Right PV | 10.5×4.5×7 | Chest tightness | PL Th | Goiter |
| Yin et al. (2018) | 68 | F | Posterior mediastinum | 13×10×10 | Dysphagia | UN | Gastrointestinal stromal tumors |
| Loftus et al. (2018) | 57 | F | Right PV | 13 | Dyspnea, dry cough | PL Th | Schwannoma |
| Tsimpinos et al. (2021) | 75 | M | Right PV | UN | Dyspnea | PL Th | Triton tumor |
| Savu et al. (2020) | 60 | M | Right PV | 20.5×12.5×9 | Dyspnea | PL Th | Benign schwannoma |
| Chen et al. (2017) | 68 | F | Post mediastinum | 13×10×10 | Weight loss | Abdomino-thoracic approach | Gastrointestinal stromal tumor |
| Mubashir et al. (2017) | 46 | M | Left PV | 12.5×8.5×7.5 | Shortness of breath | PL Th | Cystic schwannoma |
| Zahra et al. (2017) | 39 | F | Right PV | 10×7×7 | Cervical swelling | PL Th | Goiter |
| Kandakure et al. (2019) | 50 | F | Bilateral posterior mediastinum | 42×25×10 | Breathlessness, dry cough | PL Th | Liposarcoma |
| Chaudhry et al. (2016) | 28 | M | Posterior mediastinum | 12×10×5 | Dysphagia, respiratory distress | PL Th | Goiter |
| Taki et al. (2011) | 39 | M | Posterior mediastinum | 40×30×15 | Chest pain | Bilateral Th, laparotomy | Liposarcoma |
| Hayat et al. (2011) | 45 | F | Posterior mediastinum | 21×14×9 | Shortness of breath | PL Th with laminectomy | Ganglioneuroma |
| Quartey et al. (2011) | 47 | M | Posterior mediastinum | 20.5×15.5×16 | Mid-back pain | The abdominal approach | Schwannoma |
| Tanimura et al. (2008) | 24 | F | Posterior mediastinum | 27×19×11 | Severe cough | PL Th (5th and 9th intercostal spaces) | Malignant mesenchymoma |
| Bouchikh et al. (2013) | 49 | F | Posterior mediastinum | 28×20×20 | Orthopnoea, chest pain | Antero-lateral Th | Desmoid tumor |
| Kirschbaum et al. (2013) | 35 | M | Posterior mediastinum | 12×20 | Cough | Antero-lateral Th | Schwannoma |
PV, paravertebral; P, posterior; PL, posterolateral; Th, thoracotomy; UN, unknown.
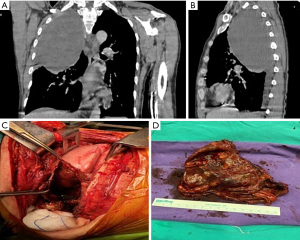
Spinal cord ischemia may develop in 3% of patients during neurogenic tumor surgery (20). In lesions proximal to the thoracolumbar (T7-L4) region, the Adamkiewicz artery, the most critical radicular artery perfusing the spinal cord, may be damaged. Loftus et al. reported that bleeding complications could be prevented by closing the main vessel (intercostal artery) that perfuses the giant posterior mediastinal tumor with angioembolization four days before the operation. They also reported that the T11 Adamkiewicz artery was preserved during the procedure (3). This artery is left-sided in 80% of patients, and it has several anatomical variations, but there is no standard pre-surgery verification method.
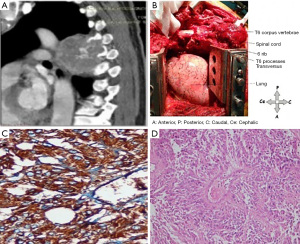
Rarely, an intraspinal approach is required to treat posterior mediastinal tumors, especially tumors of neural origin, because of their extension toward the spinal cord. Tumors that do not extend into the spinal canal and only compress the cord could be removed by careful dissection without laminectomy. However, laminectomy is inevitable in tumors that develop toward the cord or exhibit insufficient exposure (12). In our clinic, we usually apply a hockey stick incision to achieve the simultaneous exposure of the tumors (Figures 3,4). The thoracotomy is performed at a point distant from the lesion, and the laminectomy is planned based on the extent of the tumor. Mostly, the patients who undergo hemilaminectomy exhibit a fair prognosis. Generally, spinal instrumentation is not required. However, for lesions that invade more than one vertebral lamina and corpus, which are mostly lung cancer tumors, vertebral support is provided by a titanium cage. Hooks and rods are employed to support laminectomy in the posterior region. These surgeries could also be performed in two stages, depending on the degree of invasion of the lesion. In tumors with intraspinal extension, caution should be exercised in surgical manipulations to avoid serious complications, such as spinal injury, dura mater injury, and hemorrhage.
Post-surgery follow-up
In posterior mediastinal tumors, follow-up is defined by the tumor histopathology, whereas malignant tumors require adjuvant treatment and close follow-up for the early detection of local or distant recurrences, benign tumors do not (6,8,15). Although schwannoma, one of the peripheral nerve sheath tumors, may grow significantly, tumors of this kind tend to be benign and generally do not recur (18). Similarly, chemotherapy and radiotherapy are not required for treating ganglioneuromas following complete resection because of the low recurrence rate. Additionally, tumors of this kind have a fair prognosis (21). Ganglioneuroblastomas are less aggressive tumors than neuroblastomas. The first treatment choice for these tumors is surgery. Non-surgical treatment, such as chemotherapy, may lead to tumor progression (17). Although granular cell tumors from Schwann cells are benign, they rarely exhibit malignant behavior (22).
In benign tumors, local recurrence is unexpected after en bloc excision with a clear surgical margin. However, benign or malignant characteristics and histopathological subtypes should be well determined. For example, in some subtypes of Castleman’s disease, a benign entity mimics malignant diseases in histopathological features or progressive course (5). On the other hand, there is no such concern in obviously benign mediastinal masses, such as intrathoracic goiter, which are rarely observed in the posterior mediastinum (9,23).
Albeit rare, local recurrence has been reported in benign lipoma and its variant, fibrolipoma (13). Thus, follow-up should be planned according to the biological behavior of the tumor. Whereas local recurrence is 30% in well-differentiated liposarcomas after complete excision, this exceeds 50% for incomplete ones (8). Also, malignant mesenchymoma, which consists of liposarcoma and fibrosarcoma, tends to recur similarly (4). Complete surgical excision is the only treatment option in mesenchymal mediastinum tumors or mixed tumors with a sarcoma component since they are generally chemoresistant (24).
Since recurrence is rarely observed in esophageal leiomyomas, adjuvant imatinib therapy is recommended for high-risk GIST (10,25). Patients who refuse or do not tolerate adjuvant treatment should be followed up at 3-month intervals (9).
Conclusions
Tumors located in the posterior mediastinum rarely reach giant sizes. The definitive diagnosis and treatment of these tumors is surgical excision. Depending on the structures in which they originate in the posterior mediastinum or their localization characteristics, diagnostic procedures and subsequent surgical planning may change. Surgery for giant tumors of neurogenic and esophageal origins should be carefully planned, and the preoperative process should be well managed. Adjuvant treatment and follow-up should be designed considering the final histopathological features.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ryuichi Waseda and Pietro Bertoglio) for the series “Management of Giant Mediastinal Tumors” published in Mediasitinum. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://med.amegroups.com/article/view/10.21037/med-21-39/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-21-39/coif). The series “Management of Giant Mediastinal Tumors” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shields TW. The mediastinum, its compartments and the mediastinal lymph nodes. In: Shields TW, LoCicero J, Ponn RB, et al. editors. General Thoracic Surgery, vol 2, 7th ed. Philadelphia: Lippincott Williams and Wilkins, a Walters Kluwer Business, 2009:2055-8.
- Raymond DP, Daniel TM. Mediastinal Anatomy and Mediastinoscopy. In: Sellke FW, del Nido PJ, Swanson SJ. editors. Sabiston & Spencer Surgery of the Chest, 7th ed. Philadelphia: Saunders, 2005:Chapter 39.
- Loftus TJ, Pipkin M, Machuca T, et al. Angiographic embolization followed by piecemeal resection of giant posterior mediastinal schwannoma: Case report and concise review. Int J Surg Case Rep 2018;53:250-3. [Crossref] [PubMed]
- Tanimura S, Saito Y, Honma K, et al. Surgical case of giant malignant mesenchymoma in the posterior mediastinum that recurred in the bilateral mediastinum. J Nippon Med Sch 2008;75:212-5. [Crossref] [PubMed]
- Bhatti MA, Ferrante JW, Gielchinsky I, et al. Giant lymph node hyperplasia of the mediastinum (Castleman’s disease). Tex Heart Inst J 1984;11:378-84. [PubMed]
- Zahra A, Abdallah O, Farag GA. Giant Cervical Goiter With Posterior Mediastinal Extension. Cureus 2017;9:e1450. [Crossref] [PubMed]
- Chaudhry IU, Cheema AI, AlShamasi Z, et al. Hoarseness of voice, respiratory distress and dysphagia due to giant primary posterior mediastinal ectopic goitre: a rare clinical entity. BMJ Case Rep 2016;2016:bcr2016215132. [Crossref] [PubMed]
- Kandakure PR, Kambhampati S, Katta Y, et al. Giant bilateral posterior mediastinal liposarcoma excision. Indian J Thorac Cardiovasc Surg 2019;35:91-3. [Crossref] [PubMed]
- Chen H, Yao J, Tang Y, et al. A giant gastrointestinal stromal tumor of the stomach presenting as a posterior mediastinal mass. Int J Clin Exp Pathol 2017;10:8741-5. [PubMed]
- Haratake N, Shoji F, Kozuma Y, et al. Giant Leiomyoma Arising from the Mediastinal Pleura: A Case Report. Ann Thorac Cardiovasc Surg 2017;23:153-6. [Crossref] [PubMed]
- Kuzucu A, Ulutas H, Reha Celik M, et al. Hydatid cysts of the lung: lesion size in relation to clinical presentation and therapeutic approach. Surg Today 2014;44:131-6. [Crossref] [PubMed]
- Hayat J, Ahmed R, Alizai S, et al. Giant ganglioneuroma of the posterior mediastinum. Interact Cardiovasc Thorac Surg 2011;13:344-5. [Crossref] [PubMed]
- Hsu JS, Kang WY, Liu GC, et al. Giant fibrolipoma in the mediastinum: an unusual case. Ann Thorac Surg 2005;80:e10-2. [Crossref] [PubMed]
- Savu C, Grigorie V, Melinte A, et al. Giant ıntrathoracic schwannoma: A case report. In Vivo 2020;34:3527-32. [Crossref] [PubMed]
- Mubashir M, Salam A, Sonawalla A, et al. Rare Presentation of a Posterior Mediastinal Cystic Schwannoma as a Large Pleural Effusion. Cureus 2017;9:e1558. [Crossref] [PubMed]
- Taki K, Watanabe M, Iwagami S, et al. Giant liposarcoma of the posterior mediastinum and retroperitoneum. BMJ Case Rep 2011;2011:bcr0620114341. [Crossref] [PubMed]
- Karangelis D, Nikolaidis N, Roubelakis A, et al. Giant thoracoabdominal ganglioneuroblastoma in a 17-year-old patient. Asian Cardiovasc Thorac Ann 2014;22:739-41. [Crossref] [PubMed]
- Quartey B, Lenert J, Deb SJ, et al. Giant Posterior Mediastinal Ancient Schwannoma Requiring Thoracoabdominal Resection: A Case Report and Literature Review. World J Oncol 2011;2:191-4. [PubMed]
- Ryan E, Shennib H, Gopal S. Giant intrathoracic teratoma presenting with cachexia and severe dyspnea. J Cardiothorac Surg 2019;14:96. [Crossref] [PubMed]
- Furák J, Géczi T, Tiszlavicz L, et al. Postoperative paraplegia after resection of a giant posterior mediastinal tumour. Importance of the blood supply in the upper spinal cord. Interact Cardiovasc Thorac Surg 2011;12:855-6. [Crossref] [PubMed]
- Elnady B, Abdelgawaad AS, Elkhayat H. Giant intrathoracic ganglioneuroma with scoliosis treated by one-stage posterior resection and scoliosis correction: a case report. SICOT J 2020;6:12. [Crossref] [PubMed]
- De Luca G, Luciano A, Benincasa G, et al. Giant malignant granular cell tumor (GCT) of the posterior mediastinum. J Thorac Oncol 2013;8:1107-8. [Crossref] [PubMed]
- Zhao H, Ren D, Liu Y, et al. Complete transthoracic resection of giant posterior mediastinal goiter: case report and review of surgical strategies. Onco Targets Ther 2016;9:2415-9. [PubMed]
- Tsimpinos M, Pigadiotis E, Kontaxis V, et al. Giant malignant Triton tumour of the posterior mediastinum. Interact Cardiovasc Thorac Surg 2021;33:657-9. [Crossref] [PubMed]
- Yin X, Shen C, Yin Y, et al. Giant gastric stromal tumor mimicking as a posterior mediastinal mass: A case report and literature review. Medicine (Baltimore) 2018;97:e12816. [Crossref] [PubMed]
Cite this article as: Demiroz SM, Sayan M, Celik A. Giant tumors of the posterior mediastinum: a narrative review of surgical treatment. Mediastinum 2022;6:36.