
An asymptomatic giant AB thymoma in a patient with Down syndrome: a case report
Introduction
Mediastinum is the anatomical space between the lungs characterized by a complex interweaving of different organs. Large tumoral masses in this anatomical compartment often represent a medical and surgical challenge, due to common compression and/or infiltration of the surrounding structures (1,2).
Thymic epithelial tumors represent one of the most common neoplasms of mediastinum, accounting for 35% of all the anterior mediastinal masses (3,4).
According to the literature, 60% of patients are symptomatic at diagnosis, suffering from dyspnea, acute respiratory failure, dysphagia, hemodynamic failure, or signs of compression/infiltration of mediastinal structures (5-7).
Down syndrome (DS) represents, instead, a genetic aberration commonly associated with frequent infections and some malignancies, namely lymphoblastic and myeloblastic leukemia, retinoblastomas, gonadal and extragonadal germ cell tumors, due to an altered immune system, although with a lower incidence of all other solid tumors (8-10).
We described the first case of a completely asymptomatic DS patient, surgically treated at our center for a giant AB thymoma. We present the following case in accordance with the CARE reporting checklist (available at https://med.amegroups.com/article/view/10.21037/med-22-8/rc).
Case presentation
During preoperative examinations for a planned cataract surgery in a 43-year-old male affected by DS, a chest X-ray was performed in November 2017, revealing a giant mediastinal mass invading the left hemithorax (Figure 1). The preoperative computed tomography showed an expansive lesion in the anterior mediastinum of 22.6×15.5×16.5 cm, with regular margins, parenchymatous density, cystic areas, and sporadic calcifications. The mass infiltrated the diaphragm and the pericardium, dislocating the heart, main airways, and esophagus, with a clear compression of the left main bronchus and the great mediastinal vessels (Figure 1). Due to radiological left hemi-diaphragmatic elevation, left phrenic nerve infiltration was suspected.
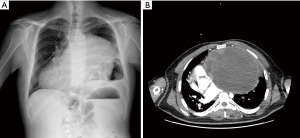
Despite the above-mentioned features, patient did not complain about any symptom, especially cardiac or respiratory ones. Lack of symptoms was confirmed by his relatives and by a dedicated full-time caregiver.
Due to common combination of DS with both lymphomas and testicular cancers, preoperative serological dosage of β2-microglobulin, lactate dehydrogenases and α-fetoprotein was performed together with the dosage of anti-acetylcholine receptor (AChR-Ab) and anti-muscle-specific kinase (MUSK-Ab) antibodies, that in our center are routinely analyzed in all subjects with a mediastinal tumor.
Results were reported in Table 1 and were suggestive for both thymoma and germinal tumor.
Table 1
| Laboratory investigation | Results (normal value) |
|---|---|
| AChR-Ab | 1.20 nmol/L (positive >0.4) |
| β2 microglobulin | 3,210.0 µg/L (200–2,000) |
| α fetoprotein | 8.7 µg/L (<7) |
Preoperative laboratory investigations. AChR-Ab, anti-acetylcholine receptor.
A core biopsy was performed in December 2017, with diagnosis of a B2 thymoma according to the World Health Organization Histologic Classification.
Due to common DS neurological and motor symptoms, other examinations as 18F-fluorodeoxyglucose-positron emission tomography (18-FDG-PET) and brain magnetic resonance imaging (MRI) were not performed due to the difficulty of the patient to remain still.
After a multidisciplinary tumor board discussion, including thoracic surgeon, oncologist, anesthesiologist, cardiologist and a neurologist, the patient was candidate for surgery in February 2018 with radical intent and the eventual prosthetic reconstruction of the organs infiltrated by the tumor.
The presence of both AChR-Ab and thymoma, together with some ocular findings including slanting of the palpebral fissures, led the neurologist to consider a stage I Myasthenia, according to the Myasthenia Gravis Foundation of America (MGFA) classification, despite neurological evaluation was extremely challenging due to the dementia and the scarce patient’s compliance. According to the dedicated neurologist’s team, steroid therapy with low dose of prednisone (12.5 mg) was administered from a month before surgery to prevent eventual myasthenic exacerbation and induce a tumor shrinkage (11).
Preoperative work-up included blood analysis to assess the hepatic and renal function, abdominal and pelvic echography to exclude any form of gonadal and extragonadal alteration, and cardiological evaluation with echocardiography, that showed a right dislocation of the heart by the mass without any signs of tamponade or neglected congenital defects.
Patient’s informed consent to the operation was acquired with the help of both his relatives and his legal guardian.
During surgery no anesthesiologic or intubation problems occurred. A complete median sternotomy was performed to achieve a large operative field and to ensure a safe dissection of the tumor from the surrounding structures. Thymoma appeared strictly adherent to the left anonymous vein, and infiltrating the left upper lobe parenchyma, the pericardium, and the left phrenic nerve. By gently dissection from the vascular structures, the mass was completely removed en-bloc with thymus, peri-thymic mediastinal fat according to the Masaoka extended thymectomy, the involved segment of left phrenic nerve, part of the anterior pericardium, a small portion of left diaphragm and almost all the left mediastinal pleura. A left Morgagni hernia was diagnosed intraoperatively, hence the diaphragmatic defect was repaired with non-absorbable suture. On the first post-operative day, a severe bleeding occurred, which required an urgent re-exploration to control the bleeding, originating from a collateral vessel of the internal left mammary vein, together with red cells and plasma transfusion. The following post-operative course was uneventful, and the patient was after ten days from surgery.
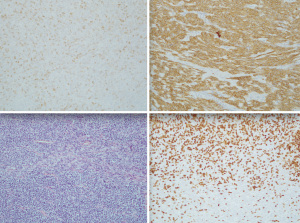
Pathology revealed an AB thymoma (Figure 2), stage IIIA according to Masaoka-Koga classification or T3N0M0 according to the TNM classification, with tumor-free margins (R0).
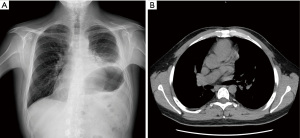
Considering histology, surgical radicality and patient’s comorbidities and inability to remain still for post-operative surgical bed radiation, the patient was candidate to exclusive clinical and radiological follow-up, with total body CT scan every six months. After a follow-up of 48 months the patient is still disease free. The 3rd year chest X-ray and the subsequent CT performed according to the surveillance plan showed no signs of local or distal recurrences (Figure 3).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient and the legal guardian for publication of this case report and the accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Giant masses of the anterior mediastinum are represented mostly by thymic tumors, germ cell tumors, thyroid goiters, and lymphomas (3,5). In this scenario, a correct preoperative diagnosis is mandatory to assess the best treatment that usually involve different specialists. Patients with chromosomic aberration as Trisomy 21 or DS, however, are mostly affected by some malignancies such as leukemias, lymphomas, germinal tumors, and retinoblastomas, while they present a globally decreased incidence of solid tumors if compared to age-adjusted with no DS controls, as reported by several authors (8-10).
In addition, Marcovecchio et al. reported that DS is usually related to an incomplete maturation of the thymus gland together with a smaller size and count of thymocytes (12).
In this case, patient developed a giant mediastinal mass, with almost complete absence of specific symptoms although with an increased serum markers value suggestive for both germinal tumor and thymoma (α-fetoprotein and AChR-Ab, respectively).
Due to the high incidence of germ cell and testicular tumors in DS, we firstly suspected a mediastinal seminoma, even though the presence of Ach-receptor raised many questions over the diagnosis (13).
To clarify any doubt a core biopsy was planned to assess the proper therapeutic process (5), revealing an AB thymoma.
Thymomas represent rare neoplasms counting for less than 1% of all malignancies in the adult population, often associated to paraneoplastic syndromes such as myasthenia gravis (MG), hypogammaglobulinemia and pure red cell aplasia (3,5,14).
According to the literature, this is the first report of a thymoma in a patient affected by DS.
Since MG is the most common autoimmune disorder associated with thymoma, mainly in type AB, B1, B2, a neurological evaluation is mandatory in all patients with a suspected thymoma. Since our patient has been diagnosed with MG, a careful perioperative control and the use of steroid therapy were strongly indicated. No other symptoms were associated to the mass (especially cardio-vascular ones) and the blood exams excluded the presence of hypogammaglobulinemia and pure red cell aplasia.
Due to thymoma’s typical slow growth, mediastinal structures generally adapt to the new condition and patients can be asymptomatic for longer periods compared to other type of tumors (15).
A complete lack of symptoms, however, is unusual for a lesion of such huge dimensions, dislocating the heart and the principal mediastinal vascular and bronchial structures. Giant mediastinal masses usually present with symptoms such as dyspnea, acute respiratory failure (especially in case of phrenic nerve involvement), dysphagia, hemodynamic failure, or other signs and symptoms of obstruction/infiltration of other mediastinal structures (known as mediastinal syndrome), in the 60% of cases (5-7).
Our case is peculiar also because the patient was affected by an outsize mass of the mediastinum dislocating all the principal mediastinal structures in absence of mediastinal syndrome.
Regarding the treatment, to date, surgery still represents a cornerstone in thymoma management both for early and advanced stages (11). In fact, these latter are often characterized by local serosal metastases and barely with lymph nodes and/or extra-thoracic spread (3-5).
Resectability depends on the extension, the anatomical relationship of the lesion with surrounding structures, as well as performance status of the patient and the presence of other comorbidity such as cardiac and respiratory impairment. Crucial is the experience of the surgeon, who assess the technical feasibility of a radical excision and the eventual need for prosthetic replacement of the infiltrated structures.
The most significant prognostic factor for both overall and disease-free survival is the radicality of the surgical resection and median sternotomy represents the most common surgical approach in case of giant masses of the anterior mediastinum, since it allows a wide surgical field and a good vision of the main mediastinal structures (1,4,7).
In this case, surgery was challenging and required the control of both the anonymous vein to gently dissect the tumor from the vessels, an en-bloc resection of the mass together with the mediastinal fat with several lymph nodes, a portion of the left upper pulmonary lobe, portions of pericardium and diaphragm and, lastly, the sacrifice of the left phrenic nerve which was englobed in the tumoral tissue. Pathology reported a stage III (according to Masaoka-Koga classification) AB thymoma with no nodal involvement, despite the core biopsy was suggestive for B2 thymoma.
This histological difference between the biopsy and the specimen may be explained by the well-known difficulty to reach a correct diagnosis in lymphocyte rich AB thymomas by analyzing core biopsies, which usually provide only a few cells with a marked alteration of the surrounding environment (16). In this stage, and for this kind of tumors, patients were usually addressed to PORT (1).
This report has a main limitation in the retrospective nature of the study, but we refined the text with an extensive and interesting discussion on the association between thymoma and DS, which has never been described in literature.
Conclusions
To date, this is the first description of a thymic malignancy in a patient with DS, that usually is characterized by a low-incidence of solid tumor except for germ-cells ones.
Large, locally advanced, thymomas resection represents a real surgical challenge due to the relationship of the mass with the neighboring organs and the high rate of associated paraneoplastic syndromes.
Notwithstanding, surgery might be considered the treatment’s cornerstone, especially in patients who could present a scarce compliance for other, local and systemic, long-lasting therapies. Cooperation between different dedicated specialists, as well as the patient’s management in highly experienced centers, represents the keystone for the success and may be always considered in such patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ryuichi Waseda and Pietro Bertoglio) for the series “Management of Giant Mediastinal Tumors” published in Mediastinum. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://med.amegroups.com/article/view/10.21037/med-22-8/rc
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-22-8/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-22-8/coif). The series “Management of Giant Mediastinal Tumors” was commissioned by the editorial office without any funding or sponsorship. ML served as an unpaid editorial board member of Mediastinum from April 2020 to March 2022. MCA served as an unpaid editorial board member of Mediastinum from March 2020 to February 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient and the legal guardian for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chiappetta M, Aprile V, Lococo F, et al. Prognostic factors for survival in advanced thymomas: The role of the number of involved structures. J Surg Oncol 2021;124:858-66. [Crossref] [PubMed]
- Aprile V, Korasidis S, Bacchin D, et al. Extended surgery of antero-superior mediastinum. Curr Chall Thorac Surg 2019;1:21. [Crossref]
- Ettinger DS, Wood EW, Aisner DL, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Thymomas and Thymic Carcinomas (version 2.2019).
- Girard N, Ruffini E, Marx A, et al. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v40-55. [Crossref] [PubMed]
- Almeida PT, Heller D. Anterior Mediastinal Mass. Updated 2021 Aug 14. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021.
- Limmer S, Merz H, Kujath P. Giant thymoma in the anterior-inferior mediastinum. Interact Cardiovasc Thorac Surg 2010;10:451-3. [Crossref] [PubMed]
- Li WW, van Boven WJ, Annema JT, et al. Management of large mediastinal masses: surgical and anesthesiological considerations. J Thorac Dis 2016;8:E175-84. [Crossref] [PubMed]
- Xavier AC, Ge Y, Taub JW. Down syndrome and malignancies: a unique clinical relationship: a paper from the 2008 william beaumont hospital symposium on molecular pathology. J Mol Diagn 2009;11:371-80. [Crossref] [PubMed]
- Satgé D, Seidel MG. The Pattern of Malignancies in Down Syndrome and Its Potential Context With the Immune System. Front Immunol 2018;9:3058. [Crossref] [PubMed]
- Hasle H, Friedman JM, Olsen JH, et al. Low risk of solid tumors in persons with Down syndrome. Genet Med 2016;18:1151-7. [Crossref] [PubMed]
- Aprile V, Korasidis S, Bacchin D, et al. Thymectomy in Myasthenic Patients With Thymoma: Killing Two Birds With One Stone. Ann Thorac Surg 2021;112:1782-9. [Crossref] [PubMed]
- Marcovecchio GE, Bortolomai I, Ferrua F, et al. Thymic Epithelium Abnormalities in DiGeorge and Down Syndrome Patients Contribute to Dysregulation in T Cell Development. Front Immunol 2019;10:447. [Crossref] [PubMed]
- Osuna-Marco MP, López-Barahona M, López-Ibor B, et al. Ten Reasons Why People With Down Syndrome are Protected From the Development of Most Solid Tumors -A Review. Front Genet 2021;12:749480. [Crossref] [PubMed]
- Guimarães MD, Benveniste MF, Bitencourt AG, et al. Thymoma originating in a giant thymolipoma: a rare intrathoracic lesion. Ann Thorac Surg 2013;96:1083-5. [Crossref] [PubMed]
- Azuma Y, Otsuka H, Makino T, et al. Giant thymoma successfully resected via median sternotomy and anterolateral thoracotomy: a case report. J Cardiothorac Surg 2018;13:26. [Crossref] [PubMed]
- Suster D, Mackinnon AC, Pihan G, et al. Lymphocyte-Rich Spindle Cell Thymoma: Clinicopathologic, Immunohistochemical, Ultrastructural and Molecular Genetic Study of 80 Cases. Am J Surg Pathol 2022;46:603-16. [Crossref] [PubMed]
Cite this article as: Sicolo E, Aprile V, Ferrarello T, Bacchin D, Mastromarino MG, Alì G, Ambrogi MC, Lucchi M, Korasidis S. An asymptomatic giant AB thymoma in a patient with Down syndrome: a case report. Mediastinum 2022;6:39.


