
A review of venous reconstruction options for the mediastinum
Introduction
Major veins, such as the superior vena cava (SVC) and the bilateral innominate veins, can make for a complicated resection of tumors that involve the structures in the mediastinum. Thymomas and non-small cell lung cancers are the most commonly encountered masses that would require a complex repair or reconstruction of these veins. Other less frequent etiologies include thymic cancer, paratracheal masses, lymphoblastoma, and carcinoid. There are numerous approaches to resection that depend on the extent of involvement of these vessels.
The operation depends on the ability to perform the appropriate oncologic resection. The approach will vary depending on the location of the mass or tumor. For mediastinal masses, a median sternotomy or clamshell incision will provide the best exposure (1). In cases of lung cancer that involve bronchoplastic or carinal resections, a muscle-sparing posterolateral thoracotomy in the fourth or fifth intercostal space would be most suitable (1). A hemi-clamshell can be used in situations of right upper lobectomy or as an alternative for mediastinal tumors. These incisions, as well as their strengths and limitations are succinctly described in chapter 2 of Adult Chest Surgery (2). Regardless of the approach, the key factor is to achieve adequate exposure for a safe oncologic operation and reconstruction. Surgeon experience, the ability to obtain proximal and distal control of the SVC or innominate veins, and the potential need for cardiopulmonary bypass (CPB) are other factors that would dictate the approach taken.
The degree of venous involvement will dictate the extent of repair or reconstruction required. There are various ways to approach the reconstruction of the SVC, innominate veins, or any combination of each structure. The use of polytetrafluoroethylene (PTFE), homograft, autologous vein, and bovine or porcine pericardial tubes all achieve the same goal of reinstituting unimpeded flow. If there is not much involvement of the venous wall, a simple repair or patch angioplasty are reasonable alternatives.
Patient selection is important in determining candidacy for such a procedure. These tumors are typically locally advanced but must be completely resectable for the patient to be a candidate for revascularization. Work-up of these patients should include computed tomography (CT) of the chest to evaluate the extent of the disease. This may be best evaluated with CT-angiography to determine vascular wall invasion. Magnetic resonance imaging (MRI) may also be of value in determining whether planes exist between structures to allow for a safe dissection.
Tissue can be obtained through fine needle transthoracic biopsy, anterior mediastinotomy, or thoracoscopically. This allows for clinical staging. Functional studies such as pulmonary function testing and physiologic cardiovascular tests should also be obtained. These operations should be approached in a controlled elective setting and never be undertaken as an urgent treatment of SVC syndrome as the morbidity and mortality rates in this situation tend to be higher.
The objective of this review is to summarize the various techniques and approaches to reconstruction of the SVC and innominate veins. Institutions around the world have different ways of treating these conditions and this chapter attempts to consolidate the information into one reference article.
Preoperative work-up and intraoperative preparation
Information regarding the extent of SVC involvement can be analyzed based on the patient’s symptomatology, physical findings, and pre-operative imaging. It is important to identify whether the SVC is completely occluded or if it has limited involvement. Total occlusion of the SVC leads to the development of an increased number of collateral vessels over time. Collateral vessels result in decreased blood flow through a conduit, increasing the risk of thrombus development (3). Being aware of an occluded SVC and choosing the appropriate conduit preoperatively can potentially decrease thrombus risk following reconstruction.
Prior to the start of the operation, venous access should be established through the femoral veins (4,5). Blood pressure should be pre-treated prior to clamping, with a goal mean arterial pressure of 80. Steroids may also be administered to decrease any cerebral edema that may occur as a consequence of clamping. Cephalic venous pressures can rise to levels of 50 mmHg during clamping (6).
Once the mediastinum is exposed, the pericardium is opened to facilitate tumor resection. Proximal dissection of the cavo-atrial junction should be performed carefully as to not injure the azygos vein (4). It is best to attempt to preserve this vessel and include it in the reconstruction if possible (4). If salvage of the azygos is not possible, some surgeons suggest ligation prior to clamping the SVC (7). Prior to clamping of the mediastinal veins, low dose heparin of 0.5 mg/kg is recommended to prevent thrombus development. Compared to clamping a chronically partially occluded SVC, the clamping of a patent SVC results in an acute SVC syndrome with the sudden drop of venous return (3). Clamping can be tolerated for 45 to 60 minutes with appropriate pharmacological support and use of ice packs (3,8). There should be constant communication with anesthesia and notification of any plethora, facial swelling, or any other signs and symptoms of worsening SVC syndrome. If occlusion of the SVC occurs for longer than 60 minutes, the risk of cerebral vein thrombosis increases. If there is concern for a prolonged clamp and sequelae, use of an intravascular shunt should be considered to allow for some flow while the area is being repaired.
Repair of the SVC
Depending on the extent of involvement of the SVC, the type of repair or reconstruction will vary. Limited involvement, without evidence of occlusion, can typically be repaired primarily. Vessel wall involvement of less than 50% can be repaired with continuous polypropylene suture on a vascular clamp (1,4,9). The larger the defect, the more likely a primary repair will result in stenosis of the vein. In this scenario, the repair can be performed with the use of a patch. There are multiple options for the type of patch that can be used in this situation. If enough autologous pericardium can be harvested, it can be shaped to the desired size for a running polypropylene repair (1,4). Another option if autologous pericardium is not feasible, can be bovine pericardium. Some surgeons have also used synthetic patches for such repairs (4). These repairs can be performed through the use of a side-biting Satinsky clamp or through a brief cross clamp.
Graft interposition with cross-clamping is preferred in cases where greater than 50% of the circumference of the vessel wall is involved (1,4). Any attempts at performing a primary repair with defects larger than this may result in kinking, thrombosis, and inevitably occlusion. If the vertical extension of tumor invasion is more than half of the total SVC length, graft reconstruction is performed (9). The most commonly documented reconstruction utilizes PTFE conduit with proximal and distal end-to-end anastomosis (Figure 1). Grafts are typically 18 to 20 mm in diameter (3,6). The SVC typically is a high flow, low pressure system. Using a graft size that is too large will result in decreased flow which increases the risk of thrombosis. Thus surgeons should carefully consider the PTFE graft size when preparing for reconstruction.
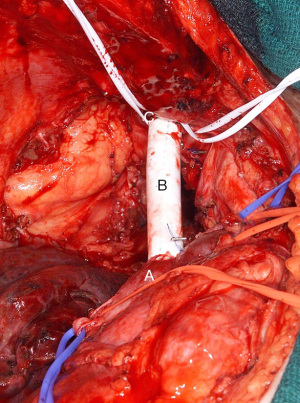
Once the SVC is cross clamped proximally and distally, the graft is sutured in place with 5-0 polypropylene running suture starting with anastomosis to the SVC stump followed by the distal anastomosis (6,8,10). If the azygos vein is being preserved, a side hole should be created allowing for an end-to-side anastomosis for the reimplantation. Before placement of the final suture, the peripheral clamp is released to allow for de-airing and release of any thrombus prior to re-establishing communication with the heart (5). Patency can be confirmed through the use of intraoperative doppler probe (5).The use of PTFE has been studied and compared to the use of bovine or porcine pericardial conduit (1,6,8). The advantages of a pericardial conduit include its biocompatibility and decreased rates of infections and thrombosis (10). Furthermore, its thinner and more pliable nature makes it easier to suture and trim to the vascular wall. Intuitive methods of creating a conduit through the use of a bovine pericardial leaflet have also been reported (Figure 2). Such a graft may be trimmed to a rectangular shape to match the area of SVC resected, wrapped around a syringe to achieve the appropriate diameter and sutured or stapled longitudinally to create a conduit (10).
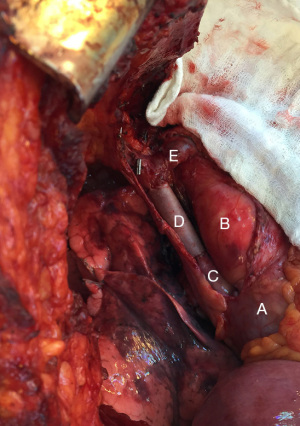
Patients that undergo PTFE reconstruction are sent home with oral anticoagulation given the increased risk of developing fibrotic tissue and becoming pro-thrombotic (1,8). Those with biologic conduits do not require anticoagulation though are often treated with antiplatelets (1,8). Primary repair or patch reconstruction does not require any anticoagulation or antiplatelet therapy (1).
Post-operatively, patients that underwent these repairs are followed up at 1 month postoperatively, and again every 4 months. A baseline CT is obtained at discharge, as well as at each follow up visit to assess patency of the graft (1). Some institutions routinely obtain CT scans at follow up visits though the timeline of these follow ups varied by surgeon. These may also correspond with any screening or surveillance imaging that may be required as part of their cancer or mass follow up. One institution obtained an MRI in their bovine pericardial group to assess the static and dynamic caliber and flow through their graft (8). The long-term patency of pericardial conduits at 1 year was 100%, while 23% of PTFE reconstructions were found to be partially or completely occluded at 6 months (8). Literature review has shown that the long-term patency rate after prosthetic reconstruction can vary widely with a reported rate of 62–100%.
The best reconstruction option will depend on the extent of repair needed. Based on the experience of multiple institutions, the best repair is a simple reconstruction with primary repair or patch though this only applies to select situations. When a complete reconstruction is required, replacement is best done with the use of pericardial conduit over PTFE given increased thrombosis and need for life-long anticoagulation with PTFE grafts.
Combination SVC and innominate vein reconstruction
Occasionally, tumor invasion may be significant enough to require reconstruction of both innominate veins and the SVC. When a tumor involves these veins, resection should be performed en-bloc (3). The initial approach to this reconstruction is similar to the isolated SVC replacement. Proximal and distal control must be obtained, with innominate veins distal to the tumor dissected and encircled with vessel loops and proximal and distal SVC dissections. Once clamping of the SVC is performed, if the venous pressure rises to greater than 40 mmHg, or if clamp time will be longer than 45 to 60 minutes, which is expected in more complicated reconstructions, it is best to place a temporary shunt (11).
When both veins are to be reconstructed, there are multiple methods to re-establishing flow. The first method described is through the use of a Y graft where both the left and right innominate veins meet into a common graft acting as a replaced SVC. The second method is to re-establish flow of innominate veins to the heart separately. When performing the Y-graft technique, it is still recommended to address each innominate vein sequentially (4).
The left innominate vein truncus is cut first, at a site allowing for adequate tumor margin. This allows creation of the left venous-graft anastomosis while the right vein continues to drain the right side (4). Once this anastomosis is completed, the SVC is cross clamped at least 1 cm below the planned site of the SVC anastomosis. This centimeter is the cuff of the vein for the graft-caval anastomosis (4). Once the left drainage is established, the right vein can be addressed by ligating and reconstructing with the Y graft to the SVC conduit (4,5).
When being addressed separately, the left innominate vein is reconstructed to the right atrial appendage with the use of an 8–10 mm graft (9). The distal end is completed first, with the graft laid as a sigmoid curve to allow for smooth blood flow and minimize the risk of kinking (9). Another option is to fix the adventitia of the graft to the right side of the ascending aorta with an interrupted 3-0 silk to prevent torsion (9). The right atrial appendage is isolated using a Satinsky clamp placed at the base. The appendage is amputated, and the trabeculae are excised to prevent the formation of thrombus from obstructed flow (5). It is important to bevel the end of the graft as the left sided conduit comes into the appendage at an angle which can result in stenosis if not matched for size. The graft is inserted to the right atrial appendage at a site measuring approximately 15 mm via the parachute technique using 4-0 polypropylene suture. Typically the right atrial appendage is used to re-establish flow of the left venous system, regardless of whether the right innominate requires reconstruction (5). Once completed, the right innominate vein may be addressed with a 10–12 mm PTFE graft anastomosed end to end to the intrapericardial SVC (9).
Use of aortic allograft has also been reported for SVC and innominate vein reconstructions (11,12). An end-to-end anastomosis is constructed using the transverse arch of a homograft, with appropriate diameter and length decided based on pre-operative CT (12). Preparation of the homograft involves excision of the aortic valve and the length of the allograft needed is measured. The proximal allograft is anastomosed to the right atrial appendage and distally to the left innominate vein. The right innominate vein is connected to the homograft through the use of the right innominate arterial homograft branch in an end-to-end fashion. As is expected, the native innominate vein is larger than the homograft innominate artery which, once again, requires a bevel to accommodate the size discrepancy (12).
Lastly, it must be stressed that the left innominate vein does not need to be reconstructed. Collateral circulation can account for drainage from the left system. Hence, leaving the left vein stapled off is an option as long as the right sided system with the SVC is reconstructed and there is no evidence of venous congestion. Furthermore, in this situation, the left IJ and subclavian vein junction should be preserved to allow cerebral venous flow to the collaterals around the left shoulder and chest.
Long term results and complications
PTFE has been shown to be durable though most institutions recommend long term anticoagulation to prevent the development of thrombosis. Some recommend the use of warfarin for a period of 6 months until endothelialization of the graft can be achieved (5). Graft thrombosis is often observed in the early postoperative phase due to technical error resulting in stenosis, kinking of the graft, or proximal venous pathology (4). This is characteristically observed with excessive dissection of the innominate vein proximally. Kinking or malrotation of the graft requires surgical correction while stenosis secondary to fibrosis can be addressed with angioplastic dilatation or stent placement (4). Acute thrombosis can result in SVC syndrome which may lead to brain damage or pulmonary embolism.
When addressing bilateral innominate veins, the patency rates will vary depending on if single or bilateral innominate veins were reconstructed. Typically, the patency of right single graft fares worse compared to those of double grafts or single left grafts (9). Comparatively, patency of the left graft was decreased compared to right in double grafts. At 12 months, single right grafts had a patency rate of 50% while double grafts showed a patency rate of 55% in the left compared to 88% on the right (9). In cases of Y-graft reconstruction with PTFE, the left innominate vein graft has been observed to be the source of thrombosis (5,11). In a group of 2 patients, both patient developed occlusion of the left innominate vein graft (11). This may be in part due to the thrombotic nature of constructing a graft to graft anastomosis or the slower flow velocity in the left innominate vein secondary to its longer length (11). If Y-graft technique is used, a short term treatment with anticoagulation may be necessary though an optimal duration has not been determined.
This increased thrombosis rate can be otherwise diminished by creating two independent conduits from each innominate vein to the right atrium or SVC. Furthermore, using two independent conduits diminishes the repercussions of an acute SVC syndrome since the contralateral system will allow for drainage of blood. Additionally, if a good right sided reconstruction of the SVC is achieved, the left sided innominate can be left closed as long as there is no evidence of venous congestion.
A disadvantage reported with the use of homografts as a conduit is the long term development of calcifications, though this does not appear to affect the patency (12). The use of homograft has not been widely reported and is based on the experience of a small subset of institutions. The patency of these grafts should be routinely assessed given the lack of long-term data.
Further complications of mediastinal vein reconstruction may include thoracic infections which will typically present as mediastinitis, empyema, or septicimia (4). This can be addressed through a washout and omentoplasty procedure to cover the graft. Though, if there is concern for bacteremia with graft infection, then graft excision should be performed with homograft replacement (4).
Conclusions
Reconstructing the veins of the mediastinum can be a challenging surgery to undertake. In the correct patient population, it can drastically improve the survival and quality of life from their disease process. The appropriate reconstruction will depend on the veins involved and the extent of vessel wall involvement. This variety includes something as simple as a primary repair without cross clamping to CPB and replacement of bilateral innominate veins and the SVC. There are numerous options to achieve this, and the choice depends largely on surgeon preference and experience.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Michael T. Jaklitsch) for the series “Venous Surgery of the Mediastinum” published in Mediastinum.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-20-70/coif). The series “Venous Surgery of the Mediastinum” was commissioned by the editorial office without any funding or sponsorship. JOW is a Consultant for Intuitive, Boston Scientific, Meditronic, and Ethicon. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Spaggiari L, Leo F, Veronesi G, et al. Superior vena cava resection for lung and mediastinal malignancies: a single-center experience with 70 cases. Ann Thorac Surg 2007;83:223-9; discussion 229-30. [Crossref] [PubMed]
- Jaklitsch MT. Chapter 2 Thoracic Incisions. In: Adult Chest Surgery. 3rd ed. McGraw-Hill, 2020:3-19.
- Dartevelle P, Chapelier A, Navajas M, et al. Replacement of the superior vena cava with polytetrafluoroethylene grafts combined with resection of mediastinal-pulmonary malignant tumors. Report of thirteen cases. J Thorac Cardiovasc Surg 1987;94:361-6. [Crossref] [PubMed]
- Jaus M, Macchiarini P. Superior vena cava and innominate vein reconstruction in thoracic malignancies: cryopreserved graft reconstruction. Semin Thorac Cardiovasc Surg 2011;23:330-5. [Crossref] [PubMed]
- Odell DD, Liao K. Superior vena cava and innominate vein reconstruction in thoracic malignancies: double-vein reconstruction. Semin Thorac Cardiovasc Surg 2011;23:326-9. [Crossref] [PubMed]
- Spaggiari L, Veronesi G, D'Aiuto M, et al. Superior vena cava reconstruction using heterologous pericardial tube after extended resection for lung cancer. Eur J Cardiothorac Surg 2004;26:649-51. [Crossref] [PubMed]
- Okereke IC, Kesler KA. Superior vena cava and innominate vein reconstruction in thoracic malignancies: single-vein reconstruction. Semin Thorac Cardiovasc Surg 2011;23:323-5. [Crossref] [PubMed]
- Maurizi G, Poggi C, D'Andrilli A, et al. Superior Vena Cava Replacement for Thymic Malignancies. Ann Thorac Surg 2019;107:386-92. [Crossref] [PubMed]
- Sekine Y, Suzuki H, Saitoh Y, et al. Prosthetic reconstruction of the superior vena cava for malignant disease: surgical techniques and outcomes. Ann Thorac Surg 2010;90:223-8. [Crossref] [PubMed]
- D'Andrilli A, Ciccone AM, Ibrahim M, et al. A new technique for prosthetic reconstruction of the superior vena cava. J Thorac Cardiovasc Surg 2006;132:192-4. [Crossref] [PubMed]
- Shintani Y, Ohta M, Minami M, et al. Long-term graft patency after replacement of the brachiocephalic veins combined with resection of mediastinal tumors. J Thorac Cardiovasc Surg 2005;129:809-12. [Crossref] [PubMed]
- Tyner J, Baradarian S, Armstrong B, et al. Bilateral Brachiocephalic Vein and Superior Vena Cava Reconstruction With an Aortic Allograft. Ann Thorac Surg 2020;109:e49-50. [Crossref] [PubMed]
Cite this article as: Poulikidis KP, Wee JO. A review of venous reconstruction options for the mediastinum. Mediastinum 2022;6:21.


