
ITMIG 2021 Tumor Board: a case of a 37-year-old man with TNM stage IVA thymoma
Case presentation
At the 11th International Thymic Malignancy Interest Group Annual Meeting, a multidisciplinary expert panel discussed the diagnosis and treatment strategies of a 37-year-old man with TNM IVA type B2B3 thymoma.
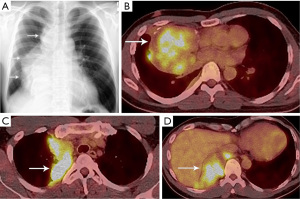
Due to pain in the right shoulder and lower chest the patient underwent chest computed tomography (CT) that revealed a right mediastinal mass and right nodular pleural thickening. Video-assisted thoracoscopic surgery (VATS) biopsy of the mass showed a thymoma (the radiological and histopathological reports from that period were unavailable to the expert panel—the diagnostics was performed at another institution). The patient received six courses of chemotherapy (CAP regimen: Cisplatin, Doxorubicin, Cyclophosphamide). The positron emission tomography (PET)/CT performed after chemotherapy did not reveal significant regression of the tumor with an 18F-fluorodeoxyglucose (FDG)-avid 6.5 cm right mediastinal mass invading the right lung and FDG-avid nodular right pleural metastases (Figure 1).
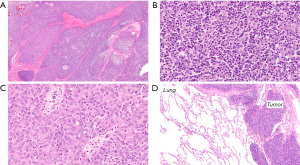
The patient contacted a center with extensive experience in the treatment of thymomas and underwent surgery through a right thoracotomy five months after chemotherapy. All neoplastic lesions from the mediastinum, the diaphragm and the lung were removed (non-anatomical resections). The pathological diagnosis of a WHO type B2B3 thymoma, TNM stage ypT3N0M1a (IVA) was established (1,2). The resection was microscopically considered incomplete (Figure 2).
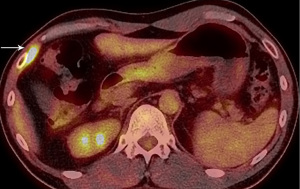
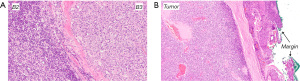
Four months later a new PET/CT showed an FDG-avid 1.5 cm recurrence in the right anterolateral lower chest wall (Figure 3) that was resected a month later. Microscopic analysis revealed a tumor of similar histology and positive resection margins (Figure 4). After surgery the patient was given three cycles of chemotherapy (Cisplatin, Ifosfamid, Etoposid).
Fifteen months after the second surgery a chest CT revealed tumor recurrence in the right mediastinal pleura and right anterior diaphragmatic space and a right upper lung lobe metastasis (Figure 5). The patient underwent right re-thoracotomy with resection of multiple lesions from the chest wall, diaphragm (resections and reconstructions), mediastinum, right pulmonary hilum and right lung (non-anatomical resections).
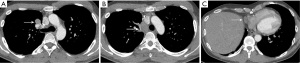
A chest CT three months later showed a 3 cm recurrence in the right anterior diaphragmatic space (Figure 6), which was removed via a subxiphoid approach.
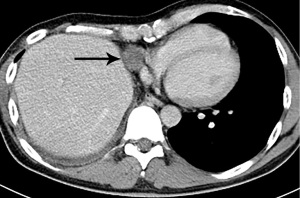
Three cycles of chemotherapy (Carboplatin, Paclitaxel) were given. The most recent chest CT four months after the last surgery showed no signs of recurrence but revealed a paralyzed right diaphragm.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Surgeon’s comments
Right thoracotomy following induction chemotherapy chosen for the initial resection of the tumor seemed to provide better access compared to a median sternotomy. The advantage of a right thoracotomy is a better view and control of pleural implants, especially along the diaphragm and deep in the costo-vertebral sulcus. Moreover, by avoiding a sternotomy, the risk of tumor spillage into the left (i.e., contralateral) pleural cavity is reduced.
However, one could also consider a right extrapleural pneumonectomy (EPP) as the initial operation rather than repeated non-anatomical resections sparing lung parenchyma (3-5). EPP might result in fewer local recurrences, but concurrently might reduce the chances for future surgical resections in case of a local pleural recurrence. This might require local radiation treatment (RT) for disease control later in the course of the disease.
The presented approach resulted so far in a successful outcome with apparently no evidence for residual disease three years after initial presentation and following multiple surgical interventions to achieve local control of the recurrent malignant thymic disease.
Radiation oncologist’s comments
There is no indication for this patient to receive (RT) at present time.
RT is predominantly used after surgery to reduce the risk of mediastinal relapse. RT can be used as a part of definitive treatment for patients who are not operable or for tumors that are not resectable after preoperative chemotherapy or for patients who are unable to tolerate the preoperative chemotherapy.
As most patients have disease confined to the thorax, RT fields often encompass one or more thoracic structures (mediastinum, pleura and, occasionally, pericardium) and is typically performed with the aim of local control and tumor eradication. For recurrent disease the doses may vary, e.g., lower doses aimed at symptoms control, while higher doses may be employed for definitive treatment (6-9). Clear statement of the clinical goal and of the area treated with radiation is needed.
Generally, patients with thymoma live long enough to manifest late side effects due to treatment. This patient has a paralyzed right diaphragm, which might compromise his respiratory and digestive systems. He needs to be followed very carefully for further recurrence and any side effects of chemotherapy he has received (10).
Medical oncologist’s comments
The probability of cure for a patient with stage IVA thymoma with surgery and chemotherapy is relatively low. There is some controversy about the role of aggressive surgical debulking. If a surgical approach is chosen then neoadjuvant chemotherapy is usually recommended, and the three-drug regimen CAP (Cisplatin, Doxorubicin, Cyclophosphamide) is frequently selected due to the high response rate and relative tolerability. In the presented case the tumor did not have a robust initial response to chemotherapy. Adjuvant chemotherapy after aggressive surgery is controversial and usually not recommended (11).
The second regimen chosen for this patient after initial recurrence, Ifosfamide, Cisplatin and Etoposide, is another aggressive combination regimen that has a high response rate. The extended period of time (well over a year) from completion of chemotherapy and the next recurrence implies some response. When the disease recurred again, the patient received Carboplatin and Paclitaxel, another known active regimen.
The risk of further progression in this patient is high (12). In case of the next recurrence, it seems reasonable to repeat the last drug regimen (carboplatin/paclitaxel) assuming there has been a long enough disease free interval to justify such an approach. Other possible therapies include the mechanistic target of rapamycin (mTOR) inhibitor—everolimus, or other chemotherapy options including pemetrexed, platinum/etoposide (the most active components of the prior regimen that led to the longest disease free interval), or gemcitabine based regimens (11). Immune checkpoint inhibitor based regimens in this patient with thymoma should be avoided given the high risk of autoimmune toxicity, but many cytotoxic chemotherapeutics have activity in thymoma. Multi-targeted tyrosine kinases inhibitors have also been utilized, though with more efficacy in thymic carcinoma than thymoma.
Systemic therapy would be the most likely next therapy, however, though more controversial, if the next recurrence was limited to the pleura there would also be some consideration for further resection followed by hyperthermic intrathoracic chemotherapy. This technique is available at limited centers, and though the data to support this approach are minimal and only retrospective, there have been some case studies with encouraging long term survival (13,14).
Conclusions
The presented case illustrates how complex and challenging the treatment of advanced thymic tumors can be and why the high stage cases require a multidisciplinary discussion.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Mediastinum for “The Series Dedicated to the 11th International Thymic Malignancy Interest Group Annual Meeting (Virtual ITMIG 2021)”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-22-3/coif). “The Series Dedicated to the 11th International Thymic Malignancy Interest Group Annual Meeting (Virtual ITMIG 2021)” was commissioned by the editorial office without any funding or sponsorship. MZ serves as an unpaid Associate Editor-in-Chief of Mediastinum from February 2020 to January 2024. ACR serves as an unpaid Associate Editor of Mediastinum from July 2021 to June 2023. MS served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Mediastinum from June 2021 to May 2023. ACR received Transplant Scholarly Time award “Unique Gene Expression Signatures in Lung Explants of Patients with Shortened Telomeres and their Prognostic Significance”, funded by Mayo Clinic Transplant Center, 2021-2023 and reported grant “Differential expression of inflammatory genes aids in the diagnosis of antibody mediated and acute rejection and their distinction from morphologic mimics in lung allografts” funded by DLMP Research Fund, 2019-2021, royalty for Contribution of medical educational material to up-to-date and honorarium from the College of American Pathologists for lecture. HW reports grants or contracts from ACEA Biosciences, Arrys Therapeutics, AstraZeneca/Medimmune BMS, Clovis Oncology Genentech/Roche, Merck, Novartis, SeaGen, Xcovery, Helsinn, participation on Advisory Boards of AstraZeneca, Xcovery, Janssen, Daiichi Sankyo, Blueprint, Mirati, Helsinn, Merck, Takeda, Genentech/Roche, Cellworks and she is a president of the International Association for the Study of Lung Cancer (IASLC) and a member of Executive committee of ECOG-ACRIN. MS reports paid lectures for Boehringer Ingelheim, AstraZeneca Pharma Poland, Roche Polska, MSD Polska and she is a Secretary of the International Thymic Malignancy Interest Group, Chair of Thymic and Mediastinal Working Group in European Society of Pathology, Member of Main Revisory Board in the Polish Society of Pathology. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Marx A, Detterbeck F, Marom EM, et al. Thymoma: In: WHO Classification of Tumours Editorial Board. Thoracic tumours [Internet]. Lyon (France): International Agency for Research on Cancer; 2021 [cited 2022-01-04]. (WHO classification of tumours series, 5th ed.; vol. 5). Available online: https://tumourclassification.iarc.who.int/chapters/35
- Rami-Porta R. Staging Manual in Thoracic Oncology, 2nd Ed. 2016 An International Association for the Study of Lung Cancer Publication. North Fort Myers, FL: Editorial Rx Press, Registered Office, 2016.
- Wright CD. Pleuropneumonectomy for the treatment of Masaoka stage IVA thymoma. Ann Thorac Surg 2006;82:1234-9. [Crossref] [PubMed]
- Fabre D, Fadel E, Mussot S, et al. Long-term outcome of pleuropneumonectomy for Masaoka stage IVa thymoma. Eur J Cardiothorac Surg 2011;39:e133-8. [Crossref] [PubMed]
- Ishikawa Y, Matsuguma H, Nakahara R, et al. Multimodality therapy for patients with invasive thymoma disseminated into the pleural cavity: the potential role of extrapleural pneumonectomy. Ann Thorac Surg 2009;88:952-7. [Crossref] [PubMed]
- Gomez D, Komaki R, Yu J, et al. Radiation therapy definitions and reporting guidelines for thymic malignancies. J Thorac Oncol 2011;6:S1743-8. [Crossref] [PubMed]
- Gomez D, Komaki R. Technical advances of radiation therapy for thymic malignancies. J Thorac Oncol 2010;5:S336-43. [Crossref] [PubMed]
- Parikh RR, Rhome R, Hug E, et al. Adjuvant Proton Beam Therapy in the Management of Thymoma: A Dosimetric Comparison and Acute Toxicities. Clin Lung Cancer 2016;17:362-6. [Crossref] [PubMed]
- Vogel J, Berman AT, Lin L, et al. Prospective study of proton beam radiation therapy for adjuvant and definitive treatment of thymoma and thymic carcinoma: Early response and toxicity assessment. Radiother Oncol 2016;118:504-9. [Crossref] [PubMed]
- NCCN. Clinical Practice Guidelines in Oncology: Thymoma and Thymic Carcinoma, Version 1.2022 - December 22, 2021. Available online: https://www.nccn.org/Home
- Girard N, Ruffini E, Marx A, et al. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v40-55. [Crossref] [PubMed]
- Chiappetta M, Lococo F, Zanfrini E, et al. The International Thymic Malignancy Interest Group Classification of Thymoma Recurrence: Survival Analysis and Perspectives. J Thorac Oncol 2021;16:1936-45. [Crossref] [PubMed]
- Yellin A, Simansky DA, Ben-Avi R, et al. Resection and heated pleural chemoperfusion in patients with thymic epithelial malignant disease and pleural spread: a single-institution experience. J Thorac Cardiovasc Surg 2013;145:83-7; discussion 87-9. [Crossref] [PubMed]
- Aprile V, Bacchin D, Korasidis S, et al. Surgical treatment of pleural recurrence of thymoma: is hyperthermic intrathoracic chemotherapy worthwhile? Interact Cardiovasc Thorac Surg 2020;30:765-72. [Crossref] [PubMed]
Cite this article as: Zielinski M, Roden AC, Truong MT, Van Raemdonck D, Komaki R, Wakelee H, Szolkowska M. ITMIG 2021 Tumor Board: a case of a 37-year-old man with TNM stage IVA thymoma. Mediastinum 2022;6:26.


