
Pyopericardium and extensive mediastinal abscess following EBUS-TBNA for mediastinal staging of NSCLC: a case report
Introduction
A precise mediastinal staging for patients with potentially resectable non-small cell lung cancer (NSCLC) takes top priority, as it provides accurate evidence of tumor stage, guides the choice of treatment and predicts the patient’s prognosis. Based on the algorithm for primary mediastinal staging, endosonographic tissue confirmation via endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is, inter alia, indicated in case of computed tomography (CT)-enlarged or positron emission tomography (PET)-positive mediastinal lymph nodes (1). It is true to the motto—“apply diagnostic tools in increasing invasiveness”—the first diagnostic choice for being both a safe minimal invasive procedure with complication rates of less than 1.5% and a valid tool with a high sensitivity defining mediastinal nodal disease (1-4). Among other peri-interventional complications—such as pneumothorax, pneumomediastinum and mediastinitis—a pyopericardium is a life-threatening condition and relativizes the apparent low complication rate in turn. If left untreated, it is rapidly progressive with mortality rates of up to 100% (5). Even when treated straight-forward, the mortality rate remain as high as 40%, mainly due to cardiac tamponade, constriction, and septic shock. With the increasingly widespread use of EBUS-TBNA, new questions arise on how to deal with such dreaded complications and—most important—how to prevent them. Focusing on this widely unknown foe, we report an impressive example of pyopericardium in conjunction with extensive mediastinal abscess following EBUS-TBNA for mediastinal staging of a NSCLC in the right upper lobe. We present the following case in accordance with the CARE reporting checklist (available at https://med.amegroups.com/article/view/10.21037/med-22-13/rc).
Case presentation
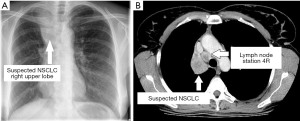
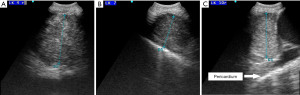
A 54-year-old woman with a 6-week history of progressive dyspnea and productive cough presented with the radiologic suspicion of a lung carcinoma of the right upper lobe (Figure 1A,1B). Relevant secondary diagnosis was chronic obstructive pulmonary disease (COPD) GOLD grade 2 with excessive nicotine consumption (almost 80 pack years). Her medical history was otherwise unremarkable. In order to receive both diagnosis and rough mediastinal staging, EBUS-TBNA target structures were—besides airway mapping biopsies—lymph node stations 4R, 7 and 10R (Figure 2A-2C). Lymph node station 7 (size: 8 mm) was punctured once using a 22G Olympus needle, lymph node station 4R (size: 17 mm) was punctured multiple times as the received samples were constantly “crumbly”, and lymph node station 10R (size 15 mm) was punctured in vain a multiple times. There was no bleeding or any other complication related to the procedure and the patient was discharged two days after the intervention with an unremarkable chest X-ray and normal laboratory findings without prophylactic antibiotics.
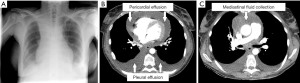
During the waiting period for histologic result, tumor board indicated PET-CT to rule out distant metastases. Unfortunately, as events turned out differently, the patient was readmitted exactly two weeks after EBUS-TBNA with symptoms of cardiogenic/septic shock: hypotension [Riva-Rocci (RR) 70/50 mmHg], tachycardia (140 beats per minute), tachypnea (35 breaths per minute), SpO2 84% without oxygen supply, chest pain and fever (39 ℃). Electrocardiogram showed low voltage and ST-segment elevation in a wide range of leads. As already presumed in chest X-ray (Figure 3A), echocardiography revealed a circumferential pericardial effusion (size 2.5 cm) with tamponade physiology. CT-scan confirmed circular pericardial effusion with an intensity scale of 27 Hounsfield units (HU), indicating a complicated protein-rich effusion (Figure 3B). In addition, a thereof separate liquid formation in the anterior mediastinum with an extension of 69×21×37 mm (intensity scale 21 HU) pointed out a possible mediastinal abscess (Figure 3C). Laboratory results were as follows: white blood cells 30.2×103/µL (cut-off <11.3×103/µL), C-reactive protein level 149.5 mg/L (cut-off <10 mg/L), and procalcitonin level 1.44 ng/mL (cut-off <0.5 ng/mL).
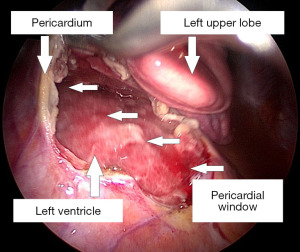
With broad-spectrum antibiotics (piperacillin-tazobactam and clindamycin), the patient underwent urgent left-sided thoracoscopic pericardial fenestration and mediastinal abscess drainage (Figure 4). Even though postoperative catecholamine dose could be initially reduced, persistent tachycardia (120 beats per minute) prompted an echocardiographic control, which revealed a relevant residual pericardial effusion in the area around right atrium and ventricle. In addition, CT-monitoring showed a still detectable mediastinal fluid collection as with a possible persistent abscess formation (Figure 5). Clindamycin was replaced by linezolid. Subsequent right-sided thoracoscopic pericardial fenestration and residual mediastinal abscess drainage was unavoidable seven days after the initial intervention. Renewed rise in infectious blood values was expression of residual purulent effusion in the right-sided pleura not being drained via the inserted chest tubes. Re-thoracoscopy for fluid discharge and further adjustment of antibiotics (linezolid replaced by meropenem) rounded the therapy off.
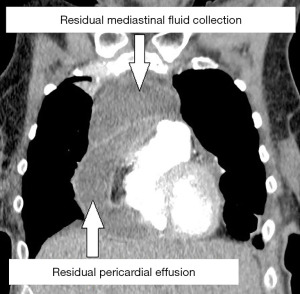
Even though cultures from various pericardial/mediastinal fluid probes remained inconspicuous, surgical intervention and antibiotic therapy were finally a breakthrough: both cardiac function and laboratory findings recovered. Meanwhile, histologic examination of the mediastinal lymph nodes revealed necrotic material with squamous cell carcinoma in lymph node station 4R. As the final chest drainage was removed 20 days after the initial operation, the patient could be discharged on day 23 after readmission.
In the successive oncologic course, PET-CT could substantiate the initial suspicion of a stage IIIA NSCLC (T2 N2 M0). To precisely determine mediastinal nodal status, video-assisted mediastinal lymphadenectomy (VAMLA) confirmed tumor-positive lymph node station 4R with extranodal extension (ENE+) and tracheal penetration. Subsequent to induction chemotherapy, the patient underwent definitive sequential chemoradiotherapy. Twelve-month follow-up confirmed stable disease.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The value of EBUS-TBNA as minimally invasive technique for mediastinal staging of NSCLC is undisputed. The risk-benefit ratio is in high favor of the procedure, but the pendulum gets out of balance when out of an approximately 1% risk of complication a specific patient suffers a 100% disaster with a potentially fatal course.
Etiology and beneficial risk factors for infectious complications are easy to comprehend. When the bronchoscope passes the oropharynx, it might get contaminated with oropharyngeal bacteria such as Streptococcus, Staphylococcus, Coryneform bacteria, Prevotella melaninogenica, Actinomyces odontolyticus, and Eikenella corrodens, inter alia (6). The aspiration needle picks up the pathogens either along its passage through the bronchoscope’s working channel or within the airway following removal of its open-ended plastic sheath during use (7). The as of now contaminated aspiration needle inoculates the oropharyngeal bacteria into the mediastinal tissue being biopsied (2,7,8). It is therefore important to note that aspirating airway secretions via the bronchoscope prior to insertion of the needle sheath will increase the risk of working channel contamination (9). But sole contamination of the bronchoscope does not seem to be the key explanation as both numbers of EBUS-TBNA interventions and numbers of documented EBUS-TBNA-associated infectious complications are diametrically opposed. It’s rather understood as prerequisite for additional risk factors.
Potential risk factor for peri-interventional mediastinitis is the puncture of necrotic or cystic structures (2,8,10). Due to the avascular environment and hence unavailable immune system of necrotic lesions or cysts, inoculation of bacteria into these structures might lead to locally uninhibited bacterial growth (7,11). It is assumed that the risk for infection is increased three-fold when necrotic lesions are biopsied (2). Against this backdrop it is advisable to thoroughly examine the chest CT-scan pre-interventionally to avoid biopsies of necrotic lesions whenever possible. If biopsies are still essential, however, both the number of times the needle punctures the lesion and the number of samples obtained per lesion should be limited (12). Associated further risk factor for contagious spread is the skill of the interventionalist.
As for the formation of mediastinitis, main risk factor for the development of a pyopericardium is linked to the act of puncture. When the aspiration needle is used in its entire extension, the pericardium might get unintentionally damaged with subsequent aspiration of the pericardial recess (7). It requires experience to unequivocally differentiate the pericardial recess from a lymph node (13). Our very skilled interventionalist targeted each of the biopsied lymph nodes with a suitable needle penetration depth, hence maintaining a safe distance from the pericardium. Retrospectively, there are two observations. First, one target structure of EBUS-TBNA was lymph node station 4R, which is the lower paratracheal lymph node on the right and located close to the superior pericardial recess. Lymph node station 4R contained a bulky necrotic lesion, and even though no direct inoculation of the pericardium is assumed to have occurred, contiguous spread might have developed by simple tissue prick of the adjacent pericardium (13). Second, the pericardium could be easily displayed while exploring lymph node station 10R, which is the hilar lymph node zone. Displaying vital structures timely ahead any attempt of taking tissue samples should become an indispensable reflex of any interventionalist.
Even though the onset of mediastinitis and/or pyopericardium is variable, most cases occurred within three weeks (median time 12.5 days) after EBUS-TBNA (12,14,15). Only one study reports a delayed onset in two patients, in whom mediastinitis occurred 40 and 53 days following EBUS-TBNA (15). Despite this exception, the patients should be thoroughly monitored for infectious complications in-between a two-week interval after the intervention. Reflecting our case, if our patient would have been sensitized to possible symptoms or would have been given the vivid advice of urgent in-hospital visit in case of discomfort, the situation would have possibly been recognized at least 5 days earlier. But some patients understate their physical condition—making any precaution futile.
The only curative treatment option is up-front surgery. Even if doing so, it feels like chasing after clinical findings—as our case strikingly shows. Although lagging behind, surgery is the savior. All the more as—not unusual—the underlying organism could not be identified on culture (16). Our patient needed three operations in the end to finally get a full debridement of all purulent cavities. The focus is on quick identification of crucial symptoms and rapid therapeutic decision-making.
Can we prevent the event of an infectious complication after EBUS-TBNA by prophylactic administration of antibiotics? Up to now no study on this focus showed any real benefit of prophylactic antibiotics in immunocompetent patients—not to mention subtleties like optimal start and duration of antibiotic treatment or the number needed to treat to prevent a peri-interventional infection (4,7). A transparent explanation for this observation is that antibiotics lack to penetrate into avascular structures such as large necrotic lymph nodes. Consequently, no international guideline—for example (I) the American Association of Bronchology and Interventional Pulmonology, (II) the American Association for Thoracic Surgery, (III) the American College of Chest Physicians, (IV) the Canadian Thoracic Society, (V) the European Association for Bronchology and Interventional Pulmonology, and (VI) the Society of Thoracic Surgeons—recommends the use of prophylactic antibiotics following EBUS-TBNA (17). Apart from patients with prosthetic heart valves, history of endocarditis, or asplenia, the prophylactic administration of peri-interventional antibiotics does rather seem to ease ones conscience than being based on evidence-based medicine (13). Another aspect is a sufficient oral hygiene before the procedure to decrease the amount of oral cavity pathogens and hence decrease the risk of infection (18). To achieve this, a possible supplementary starting point is a mouth rinse or full mouth disinfection before the procedure (9). A conceivable prospective approach in high risk patients (i.e., immunocompromised patients due to diabetes, medical treatment or being transplanted) might be—despite being not evidence-based—the combination of pre-interventional mouth rinse and peri-interventional prophylactic antibiotics (4).
Inconspicuous side effect of peri-interventional infectious complications is that nearly 20% of these patients did not underwent essential anti-cancer treatment as their general condition permits it due to prolonged therapy for infectious complications (2). Our patient—as finally being diagnosed with stage IIIA NSCLC—was suitable for curative treatment and received definitive chemoradiotherapy. Even though a delay in initiating anti-cancer treatment was recorded (interval of EBUS-TBNA to therapy), its oncologic significance remains to be seen.
Our case reports a prime example of its genres limitations. It represents a single observation and does not allow any smooth conclusions. However, it may have educational value and could help improve peri-interventional practice as it illustrates a relevant and challenging case. The complication reported represents an adverse peri-interventional event—one that surgeons refer to as happily not me instead of pointing fingers at anyone—that occurred but (at least) did not lead to patient’s permanent harm. Comprehensive reporting of potential peri-interventional complications remains a fundamental element in endoscopic interventions. Ongoing investigation of the patients’ phenotype with infectious complications after EBUS-TBNA may uncover possible causes and hence be useful for patients in need for selective prophylactic antibiotic treatment.
Recapitulating possible current strategies to prevent infectious complications after EBUS-TBNA—or at least reduce their impact: (I) pay special attention to possible necrotic texture of the designated target lesions on CT-scan; (II) strictly adhere to recommended guidelines for equipment decontamination; (III) ensure an adequate oral hygiene prior to EBUS-TBNA; (IV) guarantee a clear ultrasound vision of the puncture site; (V) limit the number of punctures; (VI) avoid biopsies of necrotic and cystic lesions; (VII) carefully monitor the patient post-interventionally to detect infectious complications as quick as possible.
Summarizing her reflections of in-hospital stay, closing remarks are from our patient: “Following my diagnosis of lung cancer I was understandably depressed. During in-hospital stay for interventional bronchoscopy, I was told that there might be effective treatment options whatsoever the extent of the tumor will be. That gave me confidence. As my physical condition worsened in the waiting period for further diagnostics, I was cautious to go hastily to hospital again due to the ongoing COVID-19 pandemic. Ultimately, with increasing deterioration my readmission was indispensably required. Never felt worse in my entire life. After the first operation I felt better, but the necessity of additional operations made me hopeless at first. I feared not to survive—but I am a fighter. In the end all worked out: it went uphill after the third operation and I fully recovered. Surgery finally saved my life. In retrospect, I was surprised how quick I convalesced. True to the motto “once I achieve this, I can achieve everything” I began chemoradiotherapy with confidence. In hindsight, I should have gone much earlier to hospital. I’m pretty sure the course of the disease would have been different”.
Conclusions
EBUS-TBNA is a well-established and valid mediastinal staging tool. With its increasing application, associated complications like infectious events are accumulating. Even though still rare, rapid diagnosis and quick surgical treatment is of utmost importance to avoid fatal outcome. As international guidelines do not recommend peri-interventional prophylactic antibiotic therapy, a thorough post-interventional monitoring of patients is an indispensable requirement.
Acknowledgments
The authors thank Dietmar Weber, MD (Department of Diagnostic and Interventional Radiology, Katholisches Klinikum Koblenz-Montabaur, Koblenz, Germany) for preparation and interpretation of Figures 1, 3 and 5.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://med.amegroups.com/article/view/10.21037/med-22-13/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-22-13/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Kang N, Shin SH, Yoo H, et al. Infectious complications of EBUS-TBNA: A nested case-control study using 10-year registry data. Lung Cancer 2021;161:1-8. [Crossref] [PubMed]
- Bante N, Singh A, Gupta A, et al. Accidental breakage of needle tip during endobronchial ultrasound-guided transbronchial needle aspiration: A case report and review of literature. Lung India 2021;38:80-3. [Crossref] [PubMed]
- Gautschi F, Opitz I, Schneiter D, et al. Mediastinitis After Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration of a Follicular Dendritic Cell Sarcoma. Arch Bronconeumol 2018;54:220-1. (Engl Ed). [PubMed]
- Patel H, Patel C, Soni M, et al. Acute Primary Pneumococcal Purulent Pericarditis With Cardiac Tamponade: A Case Report and Literature Review. Medicine (Baltimore) 2015;94:e1709. [Crossref] [PubMed]
- Liu W, Wang Y, Zhang W, et al. Pneumonia, pleurisy, mediastinitis, and mediastinal cyst infection secondary to endobronchial ultrasound-guided transbronchial needle aspiration: A case report. Medicine (Baltimore) 2021;100:e25973. [Crossref] [PubMed]
- Souma T, Minezawa T, Yatsuya H, et al. Risk Factors of Infectious Complications After Endobronchial Ultrasound-Guided Transbronchial Biopsy. Chest 2020;158:797-807. [Crossref] [PubMed]
- Yokoyama Y, Nakagomi T, Shikata D, et al. Surgical treatment for mediastinal abscess induced by endobronchial ultrasound-guided transbronchial needle aspiration: a case report and literature review. World J Surg Oncol 2017;15:130. [Crossref] [PubMed]
- Kim NY, Park JH, Park J, et al. Effect of Chlorhexidine Mouthrinse on Prevention of Microbial Contamination during EBUS-TBNA: A Study Protocol for a Randomized Controlled Trial. Tuberc Respir Dis (Seoul) 2021;84:291-8. [Crossref] [PubMed]
- Ishimoto H, Yatera K, Uchimura K, et al. A serious mediastinum abscess induced by endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA): a case report and review of the literature. Intern Med 2015;54:2647-50. [Crossref] [PubMed]
- von Bartheld MB, van Breda A, Annema JT. Complication rate of endosonography (endobronchial and endoscopic ultrasound): a systematic review. Respiration 2014;87:343-51. [Crossref] [PubMed]
- Voldby N, Folkersen BH, Rasmussen TR. Mediastinitis: A Serious Complication of Endobronchial Ultrasound-guided Transbronchial Needle Aspiration. J Bronchology Interv Pulmonol 2017;24:75-9. [Crossref] [PubMed]
- Lee HY, Kim J, Jo YS, et al. Bacterial pericarditis as a fatal complication after endobronchial ultrasound-guided transbronchial needle aspiration. Eur J Cardiothorac Surg 2015;48:630-2. [Crossref] [PubMed]
- Matsuoka K, Ito A, Murata Y, et al. Severe Mediastinitis and Pericarditis After Transbronchial Needle Aspiration. Ann Thorac Surg 2015;100:1881-3. [Crossref] [PubMed]
- Kurokawa K, Asao T, Ko R, et al. Severe mediastinitis over a month after endobronchial ultrasound-guided transbronchial needle aspiration. Respirol Case Rep 2019;7:e00426. [Crossref] [PubMed]
- Vallabhaneni S, Kichloo A, Rawan A, et al. Transbronchial Needle Aspiration Cytology and Purulent Pericarditis. J Investig Med High Impact Case Rep 2020;8:2324709620951345. [Crossref] [PubMed]
- Polkowski M, Jenssen C, Kaye P, et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline - March 2017. Endoscopy 2017;49:989-1006. [Crossref] [PubMed]
- Jang JG, Ahn JH, Lee SS. Delayed onset of mediastinitis with tracheomediastinal fistula following endobronchial ultrasound-guided transbronchial needle aspiration; A case report. Thorac Cancer 2021;12:1134-6. [Crossref] [PubMed]
Cite this article as: Hartert M, Wolf M, Huertgen M. Pyopericardium and extensive mediastinal abscess following EBUS-TBNA for mediastinal staging of NSCLC: a case report. Mediastinum 2023;7:4.


