
Asymptomatic lipofibroadenoma in a 17-year-old male: a case report and literature review of a rare entity
Introduction
The vast majority of thymic tumours fall within the category of thymoma. These are primary epithelial neoplasms often admixed with an immature lymphocytic component, thus reflecting to a certain degree organoid thymic differentiation. The World Health Organization (WHO) recognizes two thymic tumours with an adipose component, thymolipoma is considered a primary adipocytic tumour, while lipofibroadenoma (LFA) is included in the category of thymic epithelial tumours but which contains an adipocytic component (1). We here present an incidentally discovered mediastinal tumour in a young male which was resected and subsequently diagnosed as an LFA. This is to the best of our knowledge the thirteenth case of LFA reported in the literature since this entity was first described in 2001 (2) (Table 1), and the first case to be analyzed by next-generation sequencing and RNA sequencing (RNAseq). We present the following case in accordance with the CARE reporting checklist (available at https://med.amegroups.com/article/view/10.21037/med-22-32/rc).
Table 1
| No. | Authors | Year | Gender, age (years) | Symptoms, duration | Size | Associations | Follow-up [months] |
|---|---|---|---|---|---|---|---|
| 1 | Kuo and Shih (2) | 2001 | Male, 62 | Dyspnea, dizziness, PRCA | ND | B1 thymoma, PRCA | No recurrence of tumor [80], PRCA relapse 2× after removal of tumor |
| 2 | Onuki et al. (3) | 2009 | Male, 32 | Incidental finding in work-up for pneumonia, 6 months | 3 cm | – | ND |
| 3 | Wang et al. (4) | 2009 | Male, 56 | Cough, expectoration, 2 weeks | 4.5 cm | B1 thymoma | NED [24]* |
| 4 | Aydin et al. (5) | 2012 | Female, 23 | Chest pain, dyspnea, 6 months | 21 cm, 2,180 g | B1 thymoma (proposed composite B1 thymoma and lipofibroadeoma) | NED [12] |
| 5 | Qu et al. (6) | 2013 | Male, 21 | Asymptomatic, incidental finding | 10 cm | – | NED [46] |
| 6 | Makdisi et al. (7) | 2015 | Male, 20 | Acute onset of fever and cough | 23 cm, 670 g | – | NED [6] |
| 7 | Hui et al. (8) | 2018 | Male, 29 | Cough, expectoration 6 months | 6.5 cm | B1 thymoma | ND |
| 8 | Hamada et al. (9), Kurebayashi et al. (10) | 2018, 2021 | Female, 55 | Asymptomatic, incidental finding on PET-CT | 4.5 cm | Thymic hyperplasia | NED [12] |
| 9 | Kojima et al. (11) | 2018 | Male, 29 | Asymptomatic | 6 cm | – | ND |
| 10 | Hakiri et al. (12) | 2021 | Male, 28 | Asymptomatic, incidental finding | 9 cm | – | NED [6] |
| 11 | Matyjek et al. (13) | 2021 | Female, 35 | Fatigue, cough | 26 cm | ANCA-associated vasculitis with renal involvement | NED [12]; progressive renal deterioration |
| 12 | Bolca et al. (14) | 2021 | Female, 64 | Progressive dyspnea | 16 cm, 2,800 g | – | NED [48] |
| 13 | Current report | 2022 | Male, 17 | Incidental finding in work-up for pneumonia | 12.5 cm, 153 g | – | NED [13] |
*, follow-up estimated from publication date. LFA, lipofibroadenoma; PRCA, pure red cell aplasia; ND, no data; NED, no evidence of disease; PET, positron emission tomography; CT, computed tomography; ANCA, anti neutrophil cytoplasmic antibody.
Case presentation
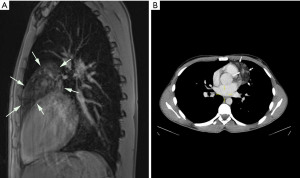
A 17-year-old previously healthy Caucasian male underwent imaging for suspected pneumonia. The magnetic resonance imaging (MRI)-scan (Figure 1A) and computed tomography (CT)-scan (Figure 1B) showed a hypointense (MRI)/hypodense (CT) tumour with focally more intense (MRI)/denser (CT) areas in the anterior mediastinum, abutting the aortic arch, pulmonary trunk and left ventricle. There was no mediastinal lymphadenopathy and there were no signs of pleural effusion. Based on the imaging investigations, the differential diagnosis included lipomatosis, lipoma/thymolipoma and liposarcoma. As serology did not reveal elevated tumour markers, a germ-cell tumour was considered unlikely. Based on the clinical and radiological findings primary thoracoscopic resection of the tumour and adjacent thymic tissue was performed through the left pleural cavity. After dissection and sealing of the feeding vessel using monopolar diathermy and a bipolar sealing device (Ligsure®, Medtronic, Minneapolis, MN, USA), the specimen was retrieved through an enlarged trocar opening using a thoracoscopic specimen retrieval bag. The recovery was uneventful. The patient was discharged on the third postoperative day.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s parent for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
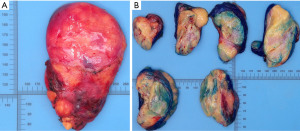
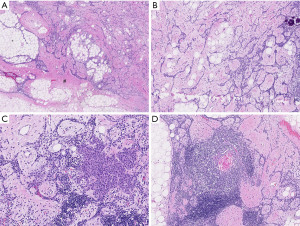
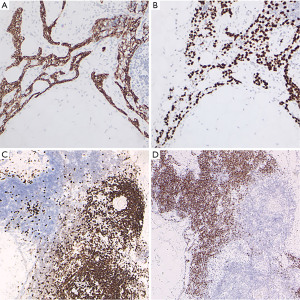
A thinly encapsulated soft tumour of 12.5×8.0×2.5 cm weighing 153 g was received (Figure 2A), which on sectioning mainly consisted of pale yellow fatty tissue with areas of pink-grey fibrous tissue (Figure 2B). Fresh tissue, checked by frozen section for the presence of representative constituents, was procured for molecular studies, the specimen was subsequently routinely fixed and processed for histology. A tumour with multiple components was seen in microscopy (Figure 3A-3D), areas of mature fat were admixed with pauci-cellular connective tissue with scattered collections of small lymphocytes. The proportion of fatty tissue to collagenous connective tissue varied considerably within the tumour (Figure 3A). Throughout these components branching interconnected cords of small epithelial cells were present (Figure 3B), occasionally associated with small solid collections of non-atypical spindled cells (Figure 3C). Scattered Hassall’s corpuscles were present (Figure 3D), typically located within lymphoid aggregates. Scattered small calcifications were noted. There was no necrosis, no atypia or nuclear hyperchromasia. Mitotic activity was extremely low. The strands and small foci of spindled cells stained for cytokeratin (pan-cytokeratin AE1-AE3) and p63 (Figure 4A,4B). The lymphoid aggregates were mainly composed of immature T-cells (CD3/TdT positive) often with small collections of CD20 positive B-cells (Figure 4C,4D). Molecular analysis was performed by whole exome sequencing (WES) and RNAseq on non-selective tumour tissue samples. For WES total DNA was isolated using the AllPrep DNA/RNA/Protein Mini Kit (Qiagen) according to standard protocol on the QiaCube (Qiagen). DNA-seq libraries were generated with 150 ng DNA using the KAPA HyperPrep Kit in combination with the HyperExome capture kit (Roche) and subsequently sequenced on an NovaSeq 6000 system (2×150 bp) (Illumina). The DNA sequencing data of the tumor and the normal (DNA extracted from blood) were processed as per the GATK 4.0 best practices workflow for variant calling, using a wdl and cromwell based workflow (https://gatk.broadinstitute.org/hc/en-us/sections/360007226651-Best-Practices-Workflows). This included performing quality control with Fastqc (version 0.11.5) to calculate the number of sequencing reads and the insert size Picard (version 2.20.1) for DNA metrics output and MarkDuplicates (15). mRNA sequencing was performed as previously described (16). In brief, total RNA was isolated using the AllPrep DNA/RNA/Protein Mini Kit (Qiagen) according to standard protocol on the QiaCube (Qiagen). RNA-seq libraries were generated with 300 ng RNA using the KAPA RNA HyperPrep Kit with RiboErase (Roche) and subsequently sequenced on an NovaSeq 6000 system (2×150 bp) (Illumina). The RNA-seq data were processed as per the GATK 4.0 best practices workflow for variant calling, using a wdl and cromwell based workflow (https://gatk.broadinstitute.org/hc/en-us/sections/360007226651-Best-Practices-Workflows). This included performing quality control with Fastqc (version 0.11.5) to calculate the number of sequencing reads and the insert size Picard (version 2.20.1) for RNA metrics output and MarkDuplicates (15). The raw sequencing reads were aligned using STAR (version 2.7.0f) to GRCh38 and gencode version 29 (16). Finally, expression counts were determined at gene level using Subread Counts (17). Fusion gene detection was performed using STARfusion.
No relevant somatic mutations or copy number variation (CNV) were detected through WES, no gene rearrangements were identified by RNAseq. Based on the findings a diagnosis of thymic LFA was made. Following surgery the patient made a good recovery and at 9 months follow-up there was no evidence of disease.
Discussion
The vast majority of thymic neoplasms are derived from thymic epithelium. Of these most are thymomas and behave as low-grade neoplasms, of which different histological subtypes are recognized in the WHO classification (1). A lymphocytic component is present in most subtypes of thymoma reflecting differentiation along the lines of the normal thymus. However, mesenchymal tissue does form part of the thymoma spectrum and as such is exceedingly rare in a primary thymic tumour, the only exception being somatic mesenchymal tissue elements in thymic germ-cell tumours. Only two primary non germ-cell thymic tumours with a mesenchymal component have been conclusively described, thymolipoma and LFA. It has been suggested that a third malignant mesenchymal (adipocytic) tumour in the mediastinum may originate in the thymus (thymoliposarcoma) (18).
Thymolipoma is a primary thymic adipocytic neoplasm consisting of mature fat which may reach a large size before producing symptoms. While scattered islands of otherwise normal thymic tissue are commonly present within the fatty tissue of thymolipoma, these are not thought to be part of the neoplasm but rather entrapped normal thymic tissue. Recently a case was described with an extensive, partly organoid, thymic epithelial component combined with areas in keeping with thymolipoma, thus blurring the border between thymoma and thymolipoma (19). Support for the neoplastic nature of thymolipoma is the identification of a HMGA-2 mutation (20). Variants of thymolipoma have been described with an excess of connective tissue, thymofibrolipoma, and with a conspicuous vascular component, thymohemangiolipoma (21-24).
In contrast to thymolipoma, LFA contains a characteristic epithelial component in addition to the lipomatous component. Therefore, in the current WHO classification thymolipoma is considered a thymic mesenchymal tumour while LFA is classified as a thymic epithelial tumour (1).
In all documented cases the epithelial component of LFA consists of small bland cells with sparse cytoplasm arranged in interconnected strands and cords ramifying through the connective tissue and fat. The typical branching epithelial strands, somewhat reminiscent of mammary fibroadenoma, prompted the designation LFA in 2001 (2). The epithelial strands are intimately associated with both the lipomatous tissue and pauci-cellular connective tissue. Features suggesting malignancy such as atypia, conspicuous proliferative activity and necrosis have not been described in LFA. The lipomatous component consists of mature fat cells devoid of lipoblasts. Pronounced calcifications were described in a single report, prompting the consideration of a germ-cell tumour (12). Small calcifications were previous described in LFA (11), and were also present in our case. In the case we present here small foci with increased cellularity were present, composed of more spindled cells, reminiscent of those seen in type A/AB thymoma. The lymphocytic component in LFA is generally poorly developed, immature TdT positive lymphocytes may be present (10-12), as in the case presented here, or may be absent (4,7-9).
Whether thymofibrolipoma and LFA are separate tumour entities or form a spectrum of composite lipomatous thymic tumours is not clear. Makdisi et al. group thymofibrolipoma together with LFA (7). Indeed, the images in the article by Moran, Zeren & Koss show histological overlap with LFA with strands of cells in fibrous tissue, suggestive of a thymofibrolipoma-LFA tumour continuum (22). However, this feature is not clearly seen in the thymofibrolipoma case presented by Kang et al. (21).
Patients with LFA are usually young (range, 17–64 years, median age 29 years), with a male predominance (4/9 female/male ratio) (2-12,14). Most LFA cases described to date were discovered incidentally by investigations performed for unrelated symptoms. If symptoms were present, these were non-specific consisting of cough or dyspnea. A single case of red cell aplasia has been reported, but in this case the LFA was associated with a B1 thymoma, which may well account for the para-neoplastic pure red cell aplasia (2).
Interestingly, in four of the hitherto described cases, the LFA was associated with a B1 thymoma, which given the rarity of both thymoma and LFA is suggestive of a pathogenetic relationship (details of reported cases in Table 1) (2,4,5,8). The follow-up of all reported cases after surgical removal of the LFA is favorable, no recurrences have been reported and patients made a good recovery. While the patient described in the report by Matyjek and co-workers made an initial good recovery from the operative procedure to remove a large LFA, she did suffer progressive anti neutrophil cytoplasmic antibody (ANCA)-vasculitis associated renal failure (13). Although it was suggested that the vasculitis may have been associated with the LFA given the co-occurrence of two rare diseases, this may be contested as the ANCA-associated vasculitis progressed after removal of the LFA. Current evidence suggests that LFA behaves in a benign fashion and only limited follow-up after removal is indicated. In those cases where as associated sub-type is present, as in the three reported cases associated with B1 thymoma it would be prudent to base the follow-up on the associated thymoma.
The nature of LFA is uncertain. The mixed composition of LFA could be taken as indicative for a hamartomatous origin rather than a true neoplasm. However, the circumscription, encapsulation and occasional large size are more in keeping with a neoplastic process. In the case presented here we did not identify genetic aberrations, specifically no HMGA-2 mutation was found, as described recently in thymolipoma, nor were CNVs documented. In a single recently described LFA case no fusion transcripts were detected (13), taken together with our case, there are currently no features which support a neoplastic process.
Conclusions
In conclusion, we report the 13th case of a rare mixed epithelial-mesenchymal tumour, LFA. The reported case bears many similarities to reported cases and adds to the awareness of this rare entity.
Acknowledgments
The authors thank Prof. Wentao Fang and Dr. Xiuxiu Hao for assisting with interpretation of the manuscript by Wang et al. (4). The assistance of Dr. Lucia Suzuki-Groenenbos and Mr. Yokichi Suzuki with the interpretation of the data in the articles by Hamada et al. (9) and Kojima et al. (11) is acknowledged.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://med.amegroups.com/article/view/10.21037/med-22-32/rc
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-22-32/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-22-32/coif). MAdB serves as an unpaid editorial board member of Mediastinum from March 2022 to February 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s parent for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours. Thoracic Tumours. 5th edition. Lyon: IARC, 2021.
- Kuo T, Shih LY. Histologic types of thymoma associated with pure red cell aplasia: a study of five cases including a composite tumor of organoid thymoma associated with an unusual lipofibroadenoma. Int J Surg Pathol 2001;9:29-35. [Crossref] [PubMed]
- Onuki T, Iguchi K, Inagaki M, et al. Lipofibroadenoma of the thymus. Kyobu Geka 2009;62:395-8. [PubMed]
- Wang YL, Yi XH, Chen G, et al. Thymoma associated with an lipofibroadenoma: report of a case. Zhonghua Bing Li Xue Za Zhi 2009;38:556-7. [PubMed]
- Aydin Y, Sipal S, Celik M, et al. A rare thymoma type presenting as a giant intrathoracic tumor: lipofibroadenoma. Eurasian J Med 2012;44:176-8. [Crossref] [PubMed]
- Qu G, Yu G, Zhang Q, et al. Lipofibroadenoma of the thymus: a case report. Diagn Pathol 2013;8:117. [Crossref] [PubMed]
- Makdisi G, Roden AC, Shen KR. Successful Resection of Giant Mediastinal Lipofibroadenoma of the Thymus by Video-Assisted Thoracoscopic Surgery. Ann Thorac Surg 2015;100:698-700. [Crossref] [PubMed]
- Hui M, Paul TR, Uppin SG, et al. Lipofibroadenoma with B1 thymoma: A case report of a rare thymic tumor. Indian J Pathol Microbiol 2018;61:630-2. [Crossref] [PubMed]
- Hamada K, Kaseda K, Omura S, et al. A case of lipofibroadenoma of the thymus. Japanese Journal of Lung Cancer 2018;58:237-8. [Crossref]
- Kurebayashi Y, Hayashi Y, Emoto K, et al. Lipofibroadenoma arising in hyperplastic thymic tissue: Possible perivascular origin of lipofibroadenoma. Pathol Int 2021;71:275-7. [Crossref] [PubMed]
- Kojima I, Matsuyama T, Tateyama H, et al. A case of lipofibroadenoma of the thymus. Pathology and Clinical Medicine 2018;36:265-9.
- Hakiri S, Kawaguchi K, Tateyama H, et al. Thymic lipofibroadenoma accompanied with largish calcifications. Gen Thorac Cardiovasc Surg 2021;69:394-7. [Crossref] [PubMed]
- Matyjek A, Stanowska O, Talarek L, et al. Giant Intrathoracic Mass in a Young Woman With Acute Kidney Injury. Chest 2021;160:e217-23. [Crossref] [PubMed]
- Bolca C, Has A, Bobocea A, et al. A Rare Thymic Tumor - Lipofibroadenoma - Always a Postoperative Surprise. In Vivo 2021;35:3623-6. [Crossref] [PubMed]
- Wingett SW, Andrews S, Fast Q. Screen: A tool for multi-genome mapping and quality control. F1000Res 2018;7:1338. [Crossref] [PubMed]
- Hehir-Kwa JY, Koudijs MJ, Verwiel ETP, et al. Improved Gene Fusion Detection in Childhood Cancer Diagnostics Using RNA Sequencing. JCO Precis Oncol 2022;6:e2000504. [Crossref] [PubMed]
- Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013;29:15-21. [Crossref] [PubMed]
- den Bakker MA, Marx A, Mukai K, et al. Mesenchymal tumours of the mediastinum--part I. Virchows Arch 2015;467:487-500. [Crossref] [PubMed]
- Szolkowska M, Blasinska K, Czajkowski W, et al. A thymoma or not a thymoma-that is the question: a case report. Mediastinum 2021;5:38. [Crossref] [PubMed]
- Hudacko R, Aviv H, Langenfeld J, et al. Thymolipoma: clues to pathogenesis revealed by cytogenetics. Ann Diagn Pathol 2009;13:185-8. [Crossref] [PubMed]
- Kang GH, Han J, Kim TS, et al. Thymofibrolipoma: A Brief Case Report. J Pathol Transl Med 2010;44:338-40.
- Moran CA, Zeren H, Koss MN. Thymofibrolipoma. A histologic variant of thymolipoma. Arch Pathol Lab Med 1994;118:281-2. [PubMed]
- Ogino S, Franks TJ, Deubner H, et al. Thymohemangiolipoma, a rare histologic variant of thymolipoma: a case report and review of the literature. Ann Diagn Pathol 2000;4:236-9. [Crossref] [PubMed]
- Anbardar MH, Amirmoezi F, Amirian A. Thymoangiolipoma: A rare histologic variant of thymolipoma in a patient with myasthenia gravis. Rare Tumors 2020;12:2036361320979215. [Crossref] [PubMed]
Cite this article as: den Bakker MA, Vermeulen MA, van de Ven CP, ter Horst SAJ, Kester L, de Krijger RR. Asymptomatic lipofibroadenoma in a 17-year-old male: a case report and literature review of a rare entity. Mediastinum 2023;7:19.


