
The anesthetic management and the role of extracorporeal membrane oxygenation for giant mediastinal tumor surgery
Introduction
Mediastinal tumors are uncommon and represent a very heterogeneous group as they are populated by benign and malignant forms. Moreover, the histological variants of tumors vary widely between the adult and pediatric populations.
The anesthetic management of patients with giant mediastinal tumors is challenging due to the non-negligible incidence of severe cardiorespiratory adverse events. In a retrospective analysis of 117 pediatric patients with mediastinal masses, the rate of anesthesia-related complications was 9.4% (1). Adult patients have a similar incidence of perioperative adverse events. Béchard and colleagues demonstrated 3.8% intraoperative cardiorespiratory complications and 10.5% postoperative respiratory complications in their analysis of 105 patients (2). In addition, general anesthesia causes physiological changes that can exacerbate cardiorespiratory collapse. Therefore, a multidisciplinary approach is mandatory from the preoperative to the postoperative phase to avoid life-threatening situations.
This review aims to provide an approach to the patient with mediastinal mass starting from the pathophysiological aspects, passing through the preoperative management, the intraoperative conduct, and the postoperative phase. In addition, we will focus on respiratory and cardiovascular complications, highlighting the role of extracorporeal membrane oxygenation (ECMO) as an integrated part of the perioperative management.
The anesthesiologist’s approach varies according to the type of planned surgical procedure. Therefore, our review aims to provide insight into the perioperative planning of patients undergoing major mediastinal surgery, leaving aside minor procedures.
Mediastinal Mass Syndrome (MMS) pathophysiology
MMS is the clinical expression of cardiorespiratory effects induced by mediastinal masses (3). These effects are enhanced by general anesthesia, which can be by itself the element of decompensation.
Respiratory complications
Respiratory complications are due to mechanical compression and/or tumor infiltration at the level of the tracheobronchial tree. Airway compression is responsible for specific signs and symptoms: cough, dyspnea, stridor, and hoarseness. The display of these symptoms is undoubtedly a warning sign for the anesthesiologist. Nevertheless, paucisymptomatic or asymptomatic patients may experience acute decompensation due to anesthesia induction as well (4). Therefore, the absence of preoperative symptoms does not exclude the risk of catastrophic intraoperative events (5). Anesthesia can exacerbate the compressive effects induced by mediastinal tumors through several mechanisms. A supine position is often required for induction of anesthesia or during surgery. It raises the compression of mediastinal structures by the gravitational effect. It also causes a cephalic displacement of the diaphragmatic dome, increasing intrathoracic pressure promoting airway compression, and impairing ventilation. Finally, the supine position increases the central blood volume, which may facilitate the volumetric increase of highly vascularised tumors. The induction of anesthesia is another critical point. The reduction of muscle tone favors the cephalad movement of the diaphragm and airway smooth muscle relaxation. This increases the risk of airway compression, mainly in the pediatric population, due to the more collapsible tissues (6).
The loss of spontaneous breathing can promote the onset of respiratory complications. Positive pressure ventilation increases pleural pressure, strengthening the compression of mediastinal structures. Poiseuille’s law states that the flow resistance is directly proportional to the fourth power of the radius when the cross-section of the airway is reduced. In this case, post-stenotic turbulent flow is produced via positive pressure ventilation. Although air can overcome the stenotic airway due to the positive pressure, it cannot be completely flushed out during the expiratory phase due to the obstruction and the lack of laminar flow, which impede gas exchange and encourage the genesis of air trapping phenomena.
Hemodynamic complications
The hemodynamic imbalance is due to compression or infiltration of the heart or major vessels (Figure 1). The compression of the pulmonary artery is uncommon, thanks to the protection provided by the tracheobronchial tree. Patients are often paucisymptomatic, sometimes presenting only exertional dyspnea. The induction of anesthesia with reduced venous return, increased pleural pressure (due to positive pressure ventilation), and the supine position may exacerbate right ventricular failure. Conversely, the superior vena cava (SVC) is more easily affected due to its location and low intravascular pressure. The obstruction of venous return from SVC generates superior vena cava syndrome (SVCS). The increased venous pressure in the upper body can result in edema of the pharynx and larynx with the onset of coughing, stridor, dyspnea, and dysphagia up to cerebral edema with headaches and mental confusion. Obstruction of venous return generates jugular turgor and in chronic forms the development of vascular networks allowing blood drainage into the inferior vena cava. SVCS occurs annually in about 15,000 people in the United States (7). Mediastinal tumors represent the most frequent malignant cause after non-small-cell and small-cell lung cancer (7). An Italian retrospective analysis of patients with stage III thymic tumors showed 24.1% involvement of major vessels with 12.4% involvement of the SVC (8). The induction of anesthesia is once again a possible cause of decompensation. Lowering the patient’s head increases hydrostatic pressure, magnifying cerebral edema. Furthermore, reducing systemic vascular resistance induced by anesthetic drugs may induce acute central hypovolemia, given the existing impairment of blood return from the SVC, which carries approximately one-third of the venous return to the heart (7).

Preoperative assessment
Clinical examination is the first step in evaluating a patient with mediastinal tumors. It is important to recognize signs and symptoms of cardio-respiratory involvement: dyspnea, coughing, stridor, hoarseness, syncope, and the patient’s upper-body edema. Furthermore, it is essential to evaluate the variation of symptoms with the patient’s position and to collect a detailed history of the patient’s preferred and non-tolerated positions. A “rescue position” should be reported during the anesthetic assessment. Patient positioning may be the simplest and most effective intraoperative maneuver to resolve life-threatening cardio-pulmonary complications. This primary assessment provides an initial grading of anesthesia risk: Anghelescu and colleagues demonstrated that orthopnea and upper body edema were significantly associated with anesthetic complications (1). Béchard and colleagues obtained similar results in adults: severe cardiorespiratory signs and symptoms were strongly associated with perioperative complications (2).
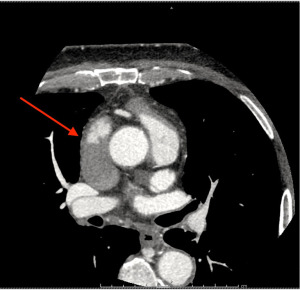
Radiological imaging is an essential adjunct in the preoperative evaluation of patients with giant mediastinal tumors. Computed tomography (CT) is the standard imaging technique. It is a rapid examination, possible to perform even in patients with poor supine tolerance. CT scan has a high sensitivity in assessing airway diameter, and the administration of contrast material allows accurate assessment of compression or thrombosis of the SVC (Figure 2). Reduction of the tracheal cross-sectional area (CSA) is a strong predictor of perioperative respiratory complications. An airway CSA, less than 50% of the standard diameter, seems to be associated with an increased risk of postoperative severe respiratory complications (2). A retrospective analysis of children with anterior mediastinal masses demonstrated a high risk of intraoperative respiratory complications in patients with either an isolated tracheal CSA less than 30% of normal or a tracheal CSA less than 70% associated with carinal or bronchial compression (9). Fiberoptic tracheobronchoscopy is another effective technique to assess airway compression or infiltration but, it should be performed in selected cases as the airway partial occlusion caused by the instrument might be itself a cause of clinical derangement. Pulmonary function tests have been extensively used in the preoperative phase, though they demonstrated a low sensitivity in the prediction of intraoperative respiratory complication. Nevertheless, Béchard and colleagues demonstrated that a peak expiratory flow of less than 40% was associated with a more than 10-fold increase in the risk of postoperative respiratory complications (2).
A comprehensive echocardiographic examination should be performed in all patients with signs or symptoms of SVCS or suggestive CT scan imaging. Echocardiographic evaluation may help excluding signs of pericardial effusion and providing information on biventricular systolic and diastolic function: good biventricular function ensures greater functional reserve in case of sudden hemodynamic changes (10). The preoperative assessment should ultimately provide a classification of anesthesia risk. We can classify patients into risk classes: safe, uncertain, and unsafe (3).
Preoperative planning is the cornerstone in managing patients with giant mediastinal masses. Perioperative discussion should involve anesthesiologists, surgeons and oncologists. All professionals should agree on the proposed treatment and alternative strategies in case of intraoperative drawbacks. Creating a multidisciplinary discussion may reduce the risk of life-threatening complications (11).
ECMO prediction
ECMO is used to treat severe cardiopulmonary failure, but it is not yet known whether it is also appropriate for use in patients with cancer. Thoracic neoplasms are distinct from other malignant tumors because they may have a direct effect on cardiopulmonary function, nevertheless as observational and registry analysis could not provide a clear recommendation pro or contra ECMO application, focus on tailored patient selection should pursued to achieve optimal results (12-14).
The preoperative phase should also identify patients at high risk of respiratory or hemodynamic collapse who could benefit from extracorporeal support. In recent years, many case reports have been published on the use of intraoperative ECMO in patients with mediastinal tumors (15,16). In addition, extracorporeal support has also been used to ensure the initial stages of chemotherapy in selected patients (17-19). The feasibility of ECMO support in cancer surgery is acknowledged (20). However, extracorporeal support is often started in emergency conditions as a rescue therapy. In 2011 a systematic review of lung resections for non–small cell lung cancer under extracorporeal support demonstrated that survival was significantly higher when placement on bypass was planned as opposed to unplanned or emergency placement (21). These data confirm the need for preoperative planning to identify patients who may benefit from extracorporeal support. Recently, Leow et al. (16) and Ramanathan and colleagues (22) proposed an algorithm to identify patients with mediastinal masses potentially eligible for ECMO. They individuated high-risk patients in the case of:
- Acute SVCS;
- Pulmonary artery or right ventricular outflow tract obstruction;
- Airway compression >50%;
- Cardiac or great vessel involvement/invasion with possible need for cardiac or vascular excision or reconstruction.
However, identifying patients with acutely decompensated SVCS who could benefit from ECMO support is not straightforward. Recently, Potere and colleagues evaluated the possible creation of a scoring system to assess the use of ECMO in patients with SVCS (23). Patients with a radiological score of II or higher (according to the classification of Qanadli et al. (24) and a grade II or higher in the symptoms classification of Yu et al. (25) should be considered candidates for ECMO (Tables 1,2).
Table 1
| Types | Degree of SVC obstruction |
|---|---|
| Type I | Stenosis of SVC up to 90% |
| Type II | 90–99% of SVC stenosis |
| Type III | Complete obstruction of SVC |
| Type IV | Complete obstruction of SVC and at least one major tributary |
SVC, superior vena cava.
Table 2
| Grade | Category | Definition |
|---|---|---|
| 0 | Asymptomatic | Radiographic signs of SVC obstruction, but no symptoms |
| 1 | Mild | Mild edema in head or neck, cyanosis or plethora |
| 2 | Moderate | Edema in head or neck with functional impairment (mild dysphagia, cough, mild or moderate impairment of head, jaw or eyelid movements, visual disturbances caused by ocular edema) |
| 3 | Severe | Mild or moderate cerebral edema (headache, dizziness) or mild/moderate laryngeal edema or diminished cardiac reserve (syncope after bending) |
| 4 | Life-threatening | Significant cerebral edema (confusion, obtundation) or significant laryngeal edema (stridor) or significant hemodynamic compromise (syncope without precipitating factors, hypotension, renal insufficiency) |
| 5 | Fatal | Death |
SVC, superior vena cava.
Moreover, high-risk patients should receive multidisciplinary planning to decide between a veno-venous or veno-arterial ECMO approach. Veno-arterial ECMO should be preferred in compromised patients with giant mediastinal masses and high risk of respiratory and hemodynamic collapse. Therefore, veno-venous ECMO should be applied in selected cases of patients with isolated respiratory symptoms and airway compression.
In all patients with at least one of the former risk factors, femoral vein/femoral artery sheaths should be inserted (depending on the type of ECMO setup chosen) before the induction of anesthesia. Perfusionists should be available in the operating room for the whole duration of surgery with a primed ECMO machine. Conversely, in patients with more than one risk factor, preoperative cannulation with the start of extracorporeal circulation before anesthetic induction should be strongly advocated.
Intraoperative management
Patients with giant mediastinal tumors need an endorsed multidisciplinary approach to achieve the best intraoperative conduct. The anesthetic approach should be tailored to the patient’s risk class and the need for possible ECMO support. Patients classified as safe should receive the same care dedicated to major thoracic surgery. Nonetheless, as already pointed out, anesthesia induction and intrathoracic pressure changes may exacerbate a cardiorespiratory collapse even in asymptomatic patients.
The anesthesiologist should escort all unsafe patients to the operating theatre. Before induction of anesthesia, a safety checklist should be follow:
- Vascular access: in all cases of SVCS, the placement of vascular accesses in tributary vessels of the SVC should be avoided to reduce thrombosis risk and to ensure the proper onset of administered drugs. The insertion of a large-bore femoral vein catheter and a femoral artery catheter should be achieved. The femoral venous catheter should be inserted distally when placing a femoral vein sheath or cannulation for ECMO or on the other limb. The risk of significant hemorrhages during mediastinal surgery dramatically increases in patients with SVCS (7,26). For this reason, the placement of a rapid infusion catheter (RIC) in a lower limb vessel is recommended.
- Airway management is always a challenge. Indeed, even without symptoms or radiological signs of airway compression, supine positioning and anesthesia induction can lead to critical airway stenosis. Therefore, the availability of various airway management devices is obliged: video laryngoscope, flexible fiberscope, rigid bronchoscope, and reinforced and double-lumen endotracheal tubes. In our opinion, well-trained thoracic anesthesiologists and experienced bronchoscopists should be available for the duration of the surgery. Moreover, perfusionists and a primed ECMO machine should always be accessible, even in unexpected cases.
- Anesthetic monitoring:
- Hemodynamic monitoring: transpulmonary thermodilution systems or pulse-contour analysis to monitor cardiac function continuously should be applied;
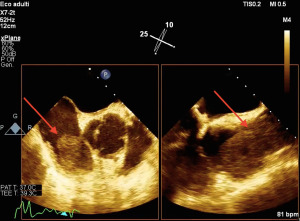
- Transesophageal echocardiography (TEE) should be used when available especially in previously evaluated patients with systolic or diastolic dysfunction, pericardial effusion, signs of heart compression or masses (Figure 3);
- Near infrared spectroscopy (NIRS) monitoring to assess any worsening of the SVCS-induced cerebral edema;
- Right arm pulse oximetry to evaluate possible injuries to the brachiocephalic trunk.
Induction of anesthesia requires a stepwise approach. Depending on local protocols and anesthesiologist’s experience, an effective way to secure the airways is awake fiberoptic intubation with the patient in spontaneous breathing. Topic anesthesia and intravenous drugs with a short half-life (remifentanil, dexmedetomidine, ketamine) guarantee the achievement of oro-tracheal intubation with an excellent level of security. Remifentanil boluses that could exacerbate stiffness, compromising patient ventilation and subsequent intubation should be avoided.
The endotracheal tube should be the largest possible to resist extrinsic compressions. We may use spiral-reinforced endotracheal or dual-lumen tubes if the surgeon requires them. Subsequently, the anesthetic plan should be deepened gradually to keep the patient breathing spontaneously. The maintenance of spontaneous breathing or pressure support ventilation avoids a significant increase in pleural pressure, decreasing the compressing effects of the mediastinal mass.
Nevertheless, as surgery often requires neuromuscular blockade, using short-acting paralyzers, such as succinylcholine or rocuronium, as its antidote; sugammadex is currently available seems a convenient choice to return to spontaneous ventilation in case of severe airway collapse or hemodynamic instability.
Recently, Hartigan and colleagues demonstrated that neuromuscular blockade did not induce airway collapse or difficult ventilation in patients with mediastinal masses presenting different grades of airway stenosis (27). Nevertheless, the study included only seventeen patients. Furthermore, patients could be excluded from the study according to the attending anesthesiologists’ choice, maybe creating a selection bias.
If the loss of spontaneous breathing exacerbates cardiorespiratory collapse in fact, the first step should be the rapid reversal of neuromuscular blockade. Meanwhile, the patient should be placed in her/his “rescue position” (if there is one), and ventilation should be attempted using a rigid bronchoscope. Unfortunately, a rigid bronchoscope can overcome tracheal compression but it hardly restores adequate ventilation in case of compression at the level of the bronchial branches.
Cardiorespiratory collapse may appear even in spontaneous breathing patients placed in their safety position. In this case, excluding a displacement of the endotracheal tube, an anesthetic plane too deep, or intraoperative bleeding, a common complication in patients with SVCS, is mandatory. Therefore, when all the reversible causes have been ruled out and treated, only two rescue therapies remain for the clinicians: the elevation of the mediastinal mass by the surgeon (sometimes with emergent sternotomy) or the extracorporeal support (veno-venous ECMO or veno-arterial ECMO). However, as already pointed out, ECMO support should be planned in the preoperative phase and not considered the last resource.
Postoperative management
Safe patients should be promptly extubated at the end of surgery and require sub-intensive care monitoring for at least 24 hours in case of no intraoperative complications.
Patients classified as unsafe or uncertain should be monitored in the intensive care unit after surgery. Achieving rapid postoperative extubation to avoid complications related to invasive ventilation (i.e., ventilator-associated pneumonia) and ensure rapid access to the rehabilitation phase or the start of chemo-radiotherapy should be pursued. Nevertheless, adequate pain management is essential to ensure rapid extubation and rehabilitation. One of the most effective approaches is multimodal and opioid-sparing analgesia (28). Several medications in combination with local anesthetics seem to have a good profile in reducing the opioids needed after thoracic surgery: ketoprofen, ketorolac, paracetamol, pethidine, flurbiprofen, dexmedetomidine (29). Among others, ketamine demonstrated a statistically significant reduction in acute post-thoracotomy pain but low power as a preventative agent for chronic post-thoracotomy pain (30) as well as pregabalin significantly reduced pain scores, decreasing postoperative neuropathic pain and morphine consumption (31).
The multimodal approach to analgesia involves regional anesthesia techniques. Thoracic epidural analgesia has been the gold standard for perioperative post-thoracotomy pain management (32). However, it is burdened by the risk of hypotension and the inability to place the epidural catheter in patients with impaired coagulation status. The search for less invasive and safer techniques is then advocated. The erector spinae plane (ESP) block might be the regional anesthesia technique of choice for the patient. It carries a low-risk profile, far from the pleura, spinal cord, and major blood vessels (32). It can be performed with high safety even in patients with impaired coagulation, and it demonstrated similar pain relief effects compared to thoracic paravertebral block (33). In a randomized controlled trial conducted on sixty patients, ultrasound-guided ESP block exhibited a significant analgesic and opioid-sparing effect in patients undergoing thoracotomy surgery (34).
Conclusions
We aimed to provide a pathophysiological view of anesthesia-induced changes in patients with giant mediastinal tumors. Understanding the physiological changes induced by anesthesia can help prevent and treat possible complications. In addition, we tried to provide an insight into different and multimodal approaches, stressing the need for a detailed preoperative phase and the need to consider extracorporeal support not as a rescue therapy but as an incorporated part of perioperative management.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Mediastinum for the series “Management of Giant Mediastinal Tumors”. The article has undergone external peer review.
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-22-35/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-22-35/coif). The series “Management of Giant Mediastinal Tumors” was commissioned by the editorial office without any funding or sponsorship. PB served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Anghelescu DL, Burgoyne LL, Liu T, et al. Clinical and diagnostic imaging findings predict anesthetic complications in children presenting with malignant mediastinal masses. Paediatr Anaesth 2007;17:1090-8. [Crossref] [PubMed]
- Béchard P, Létourneau L, Lacasse Y, et al. Perioperative cardiorespiratory complications in adults with mediastinal mass: incidence and risk factors. Anesthesiology 2004;100:826-34; discussion 5A. [Crossref] [PubMed]
- Erdös G, Tzanova I. Perioperative anaesthetic management of mediastinal mass in adults. Eur J Anaesthesiol 2009;26:627-32. [Crossref] [PubMed]
- John RE, Narang VP. A boy with an anterior mediastinal mass. Anaesthesia 1988;43:864-6. [Crossref] [PubMed]
- Viswanathan S, Campbell CE, Cork RC. Asymptomatic undetected mediastinal mass: a death during ambulatory anesthesia. J Clin Anesth 1995;7:151-5. [Crossref] [PubMed]
- Pearson JK, Tan GM. Pediatric Anterior Mediastinal Mass: A Review Article. Semin Cardiothorac Vasc Anesth 2015;19:248-54. [Crossref] [PubMed]
- Wilson LD, Detterbeck FC, Yahalom J. Clinical practice. Superior vena cava syndrome with malignant causes. N Engl J Med 2007;356:1862-9. [Crossref] [PubMed]
- Marulli G, Lucchi M, Margaritora S, et al. Surgical treatment of stage III thymic tumors: a multi-institutional review from four Italian centers. Eur J Cardiothorac Surg 2011;39:e1-7. [Crossref] [PubMed]
- Hack HA, Wright NB, Wynn RF. The anaesthetic management of children with anterior mediastinal masses. Anaesthesia 2008;63:837-46. [Crossref] [PubMed]
- Morin S, Grateau A, Reuter D, et al. Management of superior vena cava syndrome in critically ill cancer patients. Support Care Cancer 2018;26:521-8. [Crossref] [PubMed]
- Sakakura N, Nakai A, Suda H, et al. Life-threatening massive bleeding in the pulmonary trunk adjacent to the right ventricular outflow tract during the resection of a large mediastinal germ cell tumor: proposed safety measures in the absence of cardiovascular surgeons: a case report. Mediastinum 2021;5:19. [Crossref] [PubMed]
- Suzuki Y, Mao RD, Shah NR, et al. Prevalence and Impact of Infection during Extracorporeal Membrane Oxygenation in Oncologic Patients: A Retrospective Analysis of the Extracorporeal Life Support Organization (ELSO) Registry. J Intensive Care Med 2022; [Crossref] [PubMed]
- Suzuki Y, Cass S, Lentz Carvalho J, et al. Extracorporeal Membrane Oxygenation for Patients With Thoracic Neoplasms: An Extracorporeal Life Support Organization (ELSO) Registry Analysis. Ann Thorac Surg 2022;114:1816-22. [Crossref] [PubMed]
- Suzuki Y, Mobli K, Cass SH, et al. Extracorporeal Membrane Oxygenation for Adult Patients With Neoplasms: Outcomes and Trend Over the Last 2 Decades. ASAIO J 2023;69:159-66. [Crossref] [PubMed]
- Shao Y, Shen M, Ding Z, et al. Extracorporeal membrane oxygenation-assisted resection of goiter causing severe extrinsic airway compression. Ann Thorac Surg 2009;88:659-61. [Crossref] [PubMed]
- Leow L, Sampath HK, Yong KJ, et al. Rescue extracorporeal membrane oxygenation for massive anterior mediastinal masses. J Artif Organs 2021;24:450-7. [Crossref] [PubMed]
- Worku B, DeBois W, Sobol I, et al. Extracorporeal Membrane Oxygenation as a Bridge through Chemotherapy in B-Cell Lymphoma. J Extra Corpor Technol 2015;47:52-4. [PubMed]
- Lueck C, Kuehn C, Hoeper MM, et al. Successful use of extracorporeal membrane oxygenation during induction chemotherapy in a patient with mediastinal tumor mass of a T lymphoblastic lymphoma. Ann Hematol 2016;95:1719-21. [Crossref] [PubMed]
- Frey TK, Chopra A, Lin RJ, et al. A child with anterior mediastinal mass supported with veno-arterial extracorporeal membrane oxygenation. Pediatr Crit Care Med 2006;7:479-81. [Crossref] [PubMed]
- Bourcier S, Villie P, Nguyen S, et al. Venoarterial Extracorporeal Membrane Oxygenation Support Rescue of Obstructive Shock Caused by Bulky Compressive Mediastinal Cancer. Am J Respir Crit Care Med 2020;202:1181-4. [Crossref] [PubMed]
- Muralidaran A, Detterbeck FC, Boffa DJ, et al. Long-term survival after lung resection for non-small cell lung cancer with circulatory bypass: a systematic review. J Thorac Cardiovasc Surg 2011;142:1137-42. [Crossref] [PubMed]
- Ramanathan K, Leow L, Mithiran H. ECMO and adult mediastinal masses. Indian J Thorac Cardiovasc Surg 2021;37:338-43. [Crossref] [PubMed]
- Potere B, Boulos R, Awad H, et al. The Role of Extracorporeal Membrane Oxygenation in the Anesthetic Management of Superior Vena Cava Syndrome: Is it Time to Use a Scoring System? J Cardiothorac Vasc Anesth 2022;36:1777-87. [Crossref] [PubMed]
- Qanadli SD, El Hajjam M, Bruckert F, et al. Helical CT phlebography of the superior vena cava: diagnosis and evaluation of venous obstruction. AJR Am J Roentgenol 1999;172:1327-33. [Crossref] [PubMed]
- Yu JB, Wilson LD, Detterbeck FC. Superior vena cava syndrome--a proposed classification system and algorithm for management. J Thorac Oncol 2008;3:811-4. [Crossref] [PubMed]
- Dosios T, Theakos N, Chatziantoniou C. Cervical mediastinoscopy and anterior mediastinotomy in superior vena cava obstruction. Chest 2005;128:1551-6. [Crossref] [PubMed]
- Hartigan PM, Karamnov S, Gill RR, et al. Mediastinal Masses, Anesthetic Interventions, and Airway Compression in Adults: A Prospective Observational Study. Anesthesiology 2022;136:104-14. [Crossref] [PubMed]
- Long YQ, Wang D, Chen S, et al. Effect of balanced opioid-free anaesthesia on postoperative nausea and vomiting after video-assisted thoracoscopic lung resection: protocol for a randomised controlled trial. BMJ Open 2022;12:e066202. [Crossref] [PubMed]
- Andrade RR, Lima NO, Ribeiro MVMR, et al. Opioid-free postoperative analgesia compared to traditional analgesia after thoracic surgery: scoping review. Rev Assoc Med Bras (1992) 2022;68:1109-14. [PubMed]
- Moyse DW, Kaye AD, Diaz JH, et al. Perioperative Ketamine Administration for Thoracotomy Pain. Pain Physician 2017;20:173-84. [PubMed]
- Yu Y, Liu N, Zeng Q, et al. The efficacy of pregabalin for the management of acute and chronic postoperative pain in thoracotomy: a meta-analysis with trial sequential analysis of randomized-controlled trials. J Pain Res 2018;12:159-70. [Crossref] [PubMed]
- Marshall K, McLaughlin K. Pain Management in Thoracic Surgery. Thorac Surg Clin 2020;30:339-46. [Crossref] [PubMed]
- Fang B, Wang Z, Huang X. Ultrasound-guided preoperative single-dose erector spinae plane block provides comparable analgesia to thoracic paravertebral block following thoracotomy: a single center randomized controlled double-blind study. Ann Transl Med 2019;7:174. [Crossref] [PubMed]
- Sobhy MG, Abd El-Hamid AM, Elbarbary DH, et al. Ultrasound-guided erector spinae block for postoperative analgesia in thoracotomy patients: a prospective, randomized, observer-blind, controlled clinical trial. Ain-Shams Journal of Anesthesiology 2020;12:33. [Crossref]
Cite this article as: Bertini P, Marabotti A. The anesthetic management and the role of extracorporeal membrane oxygenation for giant mediastinal tumor surgery. Mediastinum 2023;7:2.


