
Intralesional microbleeding in resected thymic cysts indeterminate on imaging
Introduction
Thymic cysts are benign lesions that can be misinterpreted on computed tomography (CT) as thymic epithelial tumors (TETs) by experienced radiologists and thoracic surgeons, leading to non-therapeutic thymectomy (1).
Several studies have reported non-therapeutic thymectomy rates greater than 25% (1-3). A more recent investigation showed that a majority of unilocular thymic cysts, as defined by an index magnetic resonance imaging (MRI) examination and followed for more than 5 years, changed in volume (91%), CT attenuation (43%), and T1-weighted MRI signal intensity (67%) over time. Additionally, 16% of unique thymic cysts developed wall calcification at some point during this longitudinal study, and a majority also had CT attenuation values greater than that of water, indicating hemorrhagic and/or proteinaceous content. The thymic cysts ranged in attenuation from 0 to 100 Hounsfield units (HU). These described imaging features on CT mimic those of solid, malignant thymic neoplasms, which may explain their misinterpretation. No thin-walled unilocular thymic cyst, as initially defined by MRI, developed irregular wall thickening, mural nodularity, or septations during more than 5 years of follow-up (4). Malignant transformation of thymic cysts has been reported as extremely rare and to occur almost exclusively in multilocular thymic cystic lesions (5-10). However, review of these case reports of “transformed” multilocular thymic cysts brings to light that the occurrence of malignant transformation has been presumed, exclusively on the basis of the finding of cancer in the cyst wall. No case report that we have found provides prior imaging or other information to prove that the lesion commenced as a benign thymic cyst and subsequently transformed. In a case report describing a unilocular cystic thymic lesion that “transformed into” a papillary adenocarcinoma (5), the imaging at presentation showed the lesion to have an irregular, lobulated, asymmetrically-thickened wall on CT, features more compatible with a cystic thymoma or cystic thymic carcinoma, than a benign thymic cyst (6-9). Hence, to date and to our knowledge, there may be no proof of malignant transformation of these lesions in the literature.
We performed this study to examine the histopathological basis for the fluctuation in size and appearance of these lesions over time, which continues to baffle CT interpretation and affect surgical decision-making. We hypothesized that chronic, recurrent hemorrhage and resorption may explain the fluctuation in size, CT attenuation, and T1-weighted MRI signal of these lesions. We present the following article in accordance with the STROBE reporting checklist (available at https://med.amegroups.com/article/view/10.21037/med-22-42/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This Health Insurance Portability and Accountability Act (HIPAA)-compliant study was reviewed and approved by the Mass General Brigham (MGB) Institutional Review Board (IRB), under the protocol number 2020P000187, and informed individual consent for this retrospective analysis was waived.
Case identification
The Massachusetts General Hospital (MGH) Pathology Archives Database search for the terms “thymic cyst”, “mediastinal cyst”, and “thymic bed cyst” was performed to identify completely excised mediastinal cysts between April 2000 and May 2020. The resultant cases were reviewed, and final diagnoses were made based on histological and/or immunohistochemical (IHC) studies by a board-certified pathologist. The diagnostic hematoxylin and eosin (H&E)-stained slides, along with ancillary immunohistochemical stains, if present, were retrieved from MGH surgical pathology archives. The diagnostic criteria were based on prior published classifications of mediastinal cystic lesions (11).
Inclusion criteria for this study were: (I) a confirmed pathological diagnosis of benign thymic cyst after a microscopic assessment of the diagnostic histological material; (II) completely resected, rather than partially sampled lesions; (III) available preoperative cross-sectional imaging; and (IV) available histopathological material. Upon imaging review, cases were excluded if the epicenter of the lesion was not in the upper two-thirds of the prevascular mediastinum or if the cyst was located within, or contained, an enhancing mass.
Demographic data and clinical course
Demographic and clinical data were obtained from the MGH electronic medical record. Demographic and clinical data included age at surgery, sex, race, anatomic site, tumor size, body mass index (BMI, in kg/m²), presenting symptoms, comorbidities, clinical indication for imaging studies, and clinical indication for surgical resection.
Histopathological evaluation
Gross pathological data on the resections and hematoxylin and eosin (H&E)-stained slides included: specimen measurements, cyst dimensions (including gross maximum diameter), architectural macroscopic configuration (unilocular or multilocular), cyst fluid characteristics (when available), and nature of the excision (intact vs. disrupted or morcellated).
Prior to the microscopic evaluation of the retrieved diagnostic H&E-stained slides, a joint panel of two pathologists and a subspecialty-trained thoracic radiologist with 24 years of experience defined and selected by full consensus the histopathological features to be systematically collected in each case.
Re-analysis of the histopathological features of the cyst epithelial lining, cyst wall, surrounding thymus, and cavity contents was performed for this study by a board-certified pathologist who was blinded to clinical and radiological data. A detailed list of the microscopic findings evaluated in this study is provided in the Supplementary file (Table S1).
Imaging analysis
The CT and/or MRI performed closest to the date of surgery for each patient was reviewed on a Visage (Version 7.1.15, Build 3056) Picture Archive and Communication System (PACS) by a subspecialty-trained thoracic radiologist with 24 years of experience. The following data (Table S2) for each cyst were recorded: size—transverse, anteroposterior, and craniocaudal; morphology—round, oval, saccular, lobulated-saccular; locularity—unilocular, multilocular, indeterminate; qualitative CT attenuation relative to non-fatty chest wall musculature on the same image; quantitative CT attenuation in HU by round or elliptical (depending upon cyst shape) region-of-interest (ROI) placement over as much of the fluid component of the lesion as possible and excluding artifacts to the greatest degree possible; T1-weighted and T2-weighted MRI signal relative to muscle—hyper-, iso-, or hypointense; character of cyst wall on CT, whether thin and smooth, irregular, nodular, or a mixture of these; presence of wall enhancement on MRI (not evaluable by CT as only one CT was performed without and with intravenous (IV) contrast and wall of this cyst on CT was not discernible); maximal wall thickness on contrast-enhanced CT; maximal wall thickness on post-contrast MRI; presence of calcification; distribution of calcification—punctate or circumferential; and T1- and T2-weighted wall signal on MRI. A box listing the CT and MRI scanner hardware and software is provided in the Supplementary file (Box S1).
Statistical analysis
Descriptive summary statistics were summarized for the overall cohort. The data was analyzed using Microsoft Excel, and the results reported as mean ± one standard deviation (SD). Missing data was reported when applicable.
Results
We found a total of 85 completely resected mediastinal cyst specimens from 85 patients in the MGH Pathology Archives Database over the 20-year study period. Of these, 33 lesions were classified as thymic cysts. Upon review of the exclusion criteria, we identified 18 thymic cysts that met our pre-specified criteria. A flow diagram of the study cohort is provided in Figure 1. The nature of the remaining mediastinal cysts is provided in Table S3 of the Supplementary file.
Clinical characteristics
Patient demographics are provided in Table 1. The median age at resection was 60.5 (range, 45–77 years). The female:male ratio was 1.3. Most cysts were found incidentally (10/18, 56%) in the setting of CT imaging performed for other reasons (e.g., low-dose CT screening of smokers). Based upon preoperative imaging, only 3 cysts were classic simple cysts but were nonetheless removed (1 was incidental to concomitant coronary bypass surgery, 1 was clinically suspected to be a brachial cleft cyst, and 1 was removed due to patient preference). The other 15 patients had imaging features interpreted as most consistent with a complex cystic lesion or a thymic tumor. Cysts were often found in asymptomatic patients (8/18, 44%), but also in patients presenting with chest pain (5/18, 28%), shortness of breath (2/18, 11%), or cough (2/18, 11%).
Table 1
| Characteristics | Number of cases [%] |
|---|---|
| Age (years), mean ± SD | 60.5±9.9 |
| Sex ratio (F:M) | 1.3 |
| BMI (kg/m2), mean ± SD | 27.9±4.0 |
| Symptoms | |
| Asymptomatic | 8 [44] |
| Chest pain | 5 [28] |
| Shortness of breath | 2 [11] |
| Cough | 2 [11] |
| Other | 2 [11] |
| Concomitant conditions | |
| None | 12 [67] |
| History of thromboembolic events | 3 [17] |
| History of smoking | 3 [17] |
| Connective tissue disease | 4 [22] |
| NSIP | 1 [6] |
| Hashimoto’s thyroiditis | 2 [11] |
| Mixed connective tissue disease | 1 [6] |
| Dermatomyositis | 1 [6] |
| Primary biliary cirrhosis | 1 [6] |
| Sjögren’s | 1 [6] |
| Neoplastic | 0 |
| Other | 4 [22] |
SD, standard deviation; M, male; F, female; BMI, body mass index; NSIP, nonspecific interstitial pneumonia.
Gross pathological findings
All cysts were completely excised, but three were disrupted on macroscopic examination and, in all of these, the intracystic fluid was discarded at the time of surgery and pathologic examination and therefore unavailable for direct examination for the purpose of this study. Most cysts were unilocular (11/15, 73%), with a gross maximum diameter range of 1.5–11.2 cm (mean ± SD: 4.2±2.7 cm). When reported prior to fluid discard at time of surgery or at initial histopathologic evaluation, most cysts contained turbid-to-semi-solid, tan, hemorrhagic fluid (10/12, 83%); only two cysts (2/12, 17%) contained clear, serous-appearing fluid.
Histopathological microscopic characteristics
Diagnostic H&E-stained slides were analyzed histopathologically. Detailed histopathologic findings of the retrieved thymic cysts are described in Table 2.
Table 2
| Microscopic findings | Number of cases [%] |
|---|---|
| Findings suggestive of microbleeding and trauma | 14 [78] |
| Stromal capsular hemorrhage | 10 [55] |
| Luminal fibrin deposition | 4 [22] |
| Focal epithelial denudation | 6 [33] |
| Extracellular hemosiderin deposition | |
| In capsule | 8 [44] |
| Inside cyst cavity | 7 [39] |
| Hemosiderin-laden macrophages | 6 [33] |
| Findings suggestive of remodeling and organization | 8 [44] |
| Granulation tissue | 5 [28] |
| Histiocyte lining in areas of denudation | 2 [11] |
| Cholesterol clefts in capsule or cavity | 3 [17] |
| Foam and/or giant cells | 3 [17] |
| Acute Inflammatory infiltrate | 2 [11] |
| Findings suggestive of chronicity and scarring | 16 [89] |
| Chronic inflammatory infiltrate | 12 [67] |
| Capsular fibrosis | 12 [67] |
| Hyalinosis | 11 [61] |
| Calcifications | 6 [33] |
| Heterotopic osseous and myeloid metaplasia | 1 [6] |
| Findings in adjacent thymic tissue | 18 [100] |
| Involution | 16 [89] |
| Fat necrosis | 11 [61] |
| Microcystic Hassall’s corpuscle formation | 5 [28] |
| Lymphoid follicular hyperplasia | 3 [17] |
| True thymic hyperplasia | 1 [6] |
Findings suggestive of recent microbleeding and trauma or inflammation
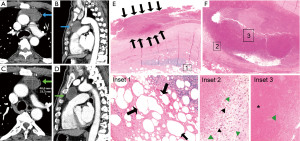
Most thymic cysts (14/18, 78%) showed findings suggestive of intralesional microbleeding, trauma, and/or inflammation in the capsule or cavity of the cyst (Figures 2,3), which included: stromal capsular hemorrhage (10/18, 55%), fibrin deposition in the lumen wall (4/18, 22%), patchy epithelial denudation (6/18, 33%), hemosiderin deposition in the capsule (8/18, 44%) or in the cavity contents (7/18, 39%), and presence of hemosiderin-laden macrophages in the cyst cavity (6/18, 33%). Additionally, most cases showed fat necrosis in the surrounding thymic tissue (12/18, 67%). Patchy necrosis and necrotic debris were also commonly seen in the capsule (7/18, 39%) and cavity (8/18, 44%), respectively.
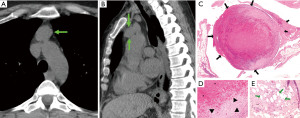
Findings suggestive of remodeling and organization
A large proportion (8/18, 44%) of thymic cysts also showed findings suggestive of recent remodeling or reorganization of their architecture (Figures 4,5), which included: presence of granulation tissue (5/18, 28%) and histiocytic lining (2/18, 11%) in areas of epithelial denudation, presence of cholesterol clefts in the capsule or cavity (3/18, 17%), presence of acute inflammatory infiltrate (2/18, 11%), and presence of foam and/or giant cells in the capsule (3/18, 17%).

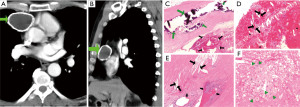
Findings suggestive of chronicity and established fibrosis
An overwhelming majority of cysts (16/18, 89%) showed findings suggestive of chronicity, pathological wound healing, and scarring of the capsule, including: presence of chronic inflammatory infiltrate (12/18, 67%), collagenous fibrosis (12/18, 67%), hyalinosis (11/18, 61%), calcifications (6/18, 33%), germinal center formation (3/18, 17%), and heterotopic osseous and myeloid metaplasia (1/18, 6%).
Additional findings in the surrounding thymus
The surrounding thymic tissue was involuted in most resections (14/18, 78%). Lymphoid thymic hyperplasia was present in three cases, and true thymic hyperplasia was seen in one case. Microcystic Hassall’s corpuscle formation was seen in a number of cases (5/18, 28%).
Imaging findings
A total of 17/18 (94%) thymic cysts were imaged preoperatively by CT, with 3/18 by both CT and MRI and 1/18 (6%) exclusively by MRI. 12/17 (70%) CTs were performed with iodinated IV contrast, 4/17 (24%) CTs were performed without IV contrast, and 1/17 (6%) was performed without and with IV contrast. All 4 (4/4) MRIs were performed without and with IV contrast (gadolinium). The mean ± SD, median, and range of the interval between preoperative imaging and surgery in days was for CT (n=17): 69±57, 49, 6–224 days and for MRI (n=4): 55±57, 53, 11–103 days.
On CT, 11/17 (65%) thymic cysts had attenuation values ≥20 HU indicative of hemorrhagic and/or proteinaceous content. A total of 6/17 (35%) cysts demonstrated wall calcification. Fifty percent (2/4) of cysts imaged by MRI were T1-isointense, 1/4 (25%) was mixed T1-hyper- and isointense, and 1/4 (25%) T1-hypointense to muscle, with latter two T1-weighted signal characteristics compatible with hemorrhagic and/or proteinaceous content. One of the four (25%) cyst walls imaged by MRI was T1/T2-hypointense, indicating presence of calcification, hemosiderin, and/or fibrosis in the wall. The accompanying CT showed wall calcification. Two of the four cysts imaged by MRI were multilocular, one of the four was unilocular, with an irregularly thickened, enhancing wall, and the fourth cyst imaged by MRI was unilocular with thin, smooth wall enhancement, though misinterpreted as solid on account of an interpretive pitfall related to a suboptimal MRI protocol in 2004. A complete tabulation of recorded CT and MRI characteristics of the thymic cysts is provided in Table S4.
Discussion
In this case series of resected thymic cysts, we report a high proportion of histopathological findings suggestive of microbleeding, remodeling, and wound healing in these lesions. The fairly common finding of adjacent fat necrosis suggests prior trauma, infection, or inflammation as an inciting agent. The T1/T2-weighted MRI signal characteristics and the CT attenuation of the majority of resected cysts were consistent with hemorrhagic and/or proteinaceous content. The irregularity and thickening of some of the walls on CT and MRI, in the setting of benign disease, therefore may have represented sequelae of chronic recurrent hemorrhage and/or inflammation. As shown in prior studies, thymic cysts can mimic thymic neoplasms on imaging (1), on account of their fluctuation in volume, CT attenuation, and MRI signal over time and on account of wall thicknesses exceeding 3 mm (4). However, the cause of these changes has not been investigated until now.
As most of our cases showed evidence of recent microbleeding along the luminal surface of the cyst, increases in volume of the total cyst fluid due to blood spillage into the cyst cavity could hypothetically increase the intraluminal pressure, further increasing the wall tension of the cyst and precipitating more bleeding and remodeling. Spontaneous bleeding within the thymus has been reported in a previously healthy patient with sudden-onset left-side chest pain, in which a thymic cystic mass progressively enlarged 18 days after initial imaging at presentation, prompting surgical resection, The resected specimen demonstrated an enlarged hemorrhagic thymus with a hemorrhagic cavity. Microscopic examination revealed a normal involuted thymus with scattered thymic tissue and significant hemorrhage in the medulla (12). As several studies have demonstrated that mechanical tension drives tissue remodeling through different mechanisms, including fibroblast-to-myofibroblast transition (13,14), we hypothesize that continuous stress to the wall of thymic cysts with intralesional bleeding could lead to tissue remodeling, fibrosis, and subsequent resorption. As expected, we found histological evidence of tissue remodeling (8/18, 44%) and/or pathological wound healing (16/18, 89%) in an overwhelming majority of cysts, which may explain the fluctuation in size, CT attenuation, and MRI signal of these lesions over time and which correlated with the presence of wall calcification on CT in some of the cysts in this study and others (4). Similar fluctuations in benign cyst size have also been reported in other organs such as the adrenal glands, in which resorption has also been reported (15).
The cause of initial bleeding in the lesions is unknown. It may be due to both external (mechanical forces) and internal (architectural abnormalities) causes. Interestingly, most of our cases (67%) showed fat necrosis in the surrounding thymic tissue, which has been associated with mechanical trauma, infection, and inflammation in other tissue types of the body (16,17).
Based on these findings and those of preceding literature (1,4,18), we propose an updated clinical management algorithm for the finding of an indeterminate round or saccular, well-circumscribed, homogeneous attenuation thymic nodule or mass measuring less than or equal to 100 HU on CT (Figure 6).
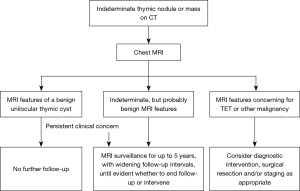
Our study has several limitations. First, it is retrospective. Second, there is selection bias which likely yielded a greater proportion of cysts with hemorrhagic findings than reflect true prevalence—only resected lesions were examined. Third, preoperative imaging was not performed on the same day as the surgery, hence more precise correlation between imaging findings and histopathology could not be made. Fourth, the presence of the histological findings of microbleeding in this study may not necessarily imply causality.
Conclusions
We report that a large proportion of completely resected thymic cysts exhibited histopathological evidence of microbleeding, remodeling, and wound healing; findings which correlated with preoperative imaging. Clinicians and pathologists involved in the diagnosis of these lesions should be aware that the variable imaging characteristics and behavior of thymic cysts over time may be secondary to intralesional bleeding and resorption and that these features, in the absence of other concerning imaging features, can be benign. If clinical concern remains regarding the imaging findings of a probably benign, but indeterminate cystic thymic lesion, MRI surveillance could be undertaken to confirm benign behavior over time, with the aim to reduce the rate of non-therapeutic thymectomy.
Acknowledgments
We thank Haley Martin and Adriana Alvarez for assisting with administrative tasks and with the biobank slide repository of the Department of Pathology at Massachusetts General Hospital. Meeting Presentation: the study was presented at the 11th International Thymic Malignancy Interest Group (ITMIG) Annual Meeting in 2021.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://med.amegroups.com/article/view/10.21037/med-22-42/rc
Data Sharing Statement: Available at https://med.amegroups.com/article/view/10.21037/med-22-42/dss
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-22-42/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-22-42/coif). JBA serves as an unpaid editorial board member of Mediastinum from July 2021 to June 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the Mass General Brigham Institutional Review Board, under the protocol number 2020P000187, and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ackman JB, Verzosa S, Kovach AE, et al. High rate of unnecessary thymectomy and its cause. Can computed tomography distinguish thymoma, lymphoma, thymic hyperplasia, and thymic cysts? Eur J Radiol 2015;84:524-33. Erratum in: Eur J Radiol 2017;90:262-3. [Crossref] [PubMed]
- Jurado J, Javidfar J, Newmark A, et al. Minimally invasive thymectomy and open thymectomy: outcome analysis of 263 patients. Ann Thorac Surg 2012;94:974-81; discussion 981-2. [Crossref] [PubMed]
- Kent MS, Wang T, Gangadharan SP, et al. What is the prevalence of a "nontherapeutic" thymectomy? Ann Thorac Surg 2014;97:276-82; discussion 82. [Crossref] [PubMed]
- Ackman JB, Chintanapakdee W, Mendoza DP, et al. Longitudinal CT and MRI Characteristics of Unilocular Thymic Cysts. Radiology 2021;301:443-54. [Crossref] [PubMed]
- Zaitlin N, Rozenman J, Yellin A. Papillary adenocarcinoma in a thymic cyst: a pitfall of thoracoscopic excision. Ann Thorac Surg 2003;76:1279-81. [Crossref] [PubMed]
- Weissferdt A, Moran CA. Thymic carcinoma associated with multilocular thymic cyst: a clinicopathologic study of 7 cases. Am J Surg Pathol 2011;35:1074-9. [Crossref] [PubMed]
- Hattori H. High-grade thymic carcinoma other than basaloid or mucoepidermoid type could be associated with multilocular thymic cyst: report of two cases. Histopathology 2003;43:501-2. [Crossref] [PubMed]
- Leong AS, Brown JH. Malignant transformation in a thymic cyst. Am J Surg Pathol 1984;8:471-5. [Crossref] [PubMed]
- Suster S, Rosai J. Multilocular thymic cyst: an acquired reactive process. Study of 18 cases. Am J Surg Pathol 1991;15:388-98. [Crossref] [PubMed]
- Singhal M, Lal A, Srinivasan R, et al. Thymic carcinoma developing in a multilocular thymic cyst. J Thorac Dis 2012;4:512-5. [PubMed]
- Goldblum JR, Lamps LW, McKenney JK, et al. Thoracic pathology. In: Rosai and Ackerman’s Surgical Pathology. Elsevier, 2018:1843-7.
- Sakuraba M, Tanaka A, Tsuji T, et al. Spontaneous thymic hemorrhage in an adult. Ann Thorac Surg 2014;97:1800-2. [Crossref] [PubMed]
- Hinz B, Mastrangelo D, Iselin CE, et al. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol 2001;159:1009-20. [Crossref] [PubMed]
- Saucerman JJ, Tan PM, Buchholz KS, et al. Mechanical regulation of gene expression in cardiac myocytes and fibroblasts. Nat Rev Cardiol 2019;16:361-78. [Crossref] [PubMed]
- Ricci Z, Chernyak V, Hsu K, et al. Adrenal cysts: natural history by long-term imaging follow-up. AJR Am J Roentgenol 2013;201:1009-16. [Crossref] [PubMed]
- Vasei N, Shishegar A, Ghalkhani F, et al. Fat necrosis in the Breast: A systematic review of clinical. Lipids Health Dis 2019;18:139. [Crossref] [PubMed]
- Song CT, Teo I, Song C. Systematic review of seat-belt trauma to the female breast: a new diagnosis and management classification. J Plast Reconstr Aesthet Surg 2015;68:382-9. [Crossref] [PubMed]
- Expert Panel on Thoracic Imaging. ACR Appropriateness Criteria® Imaging of Mediastinal Masses. J Am Coll Radiol 2021;18:S37-51. [Crossref] [PubMed]
Cite this article as: Villalba JA, Haramati A, Garlin M, Reyes F, Wright CD, Louissaint A Jr, Ackman JB. Intralesional microbleeding in resected thymic cysts indeterminate on imaging. Mediastinum 2023;7:13.


