
Airway stenting for central airway obstruction: a review
Introduction
Central airway obstruction refers to blockage of the trachea and main stem bronchi (1). The lobar bronchi are sometimes incorporated by this definition (2). Malignant causes are most often lung cancer though other malignancies metastatic to the lung can also lead to airway obstruction. Non-malignant causes include post-intubation or post-tracheostomy stenoses or those related to post-lung transplant healing; defects in the airway wall and autoimmune disease can also lead to this (3).
Various interventions for central airway obstruction exist in the armamentarium of the interventional pulmonologist, including ablative tools, mechanical debulking, and airway stents. The latter are typically employed when more conservative techniques have proven inadequate. In addition, stenting requires appropriate training and experience which then allows the proceduralist to properly select patients and the stent to be employed. Expertise in this area also prepares the physician to foresee and manage complications.
In this article, we will review airway stenting for central airway obstruction, with particular attention to the indications and patient and stent selection. We will also review the more apropos data in the literature regarding stents and their outcomes, including complications. We will then conclude by discussing new research horizons in this field.
Indications
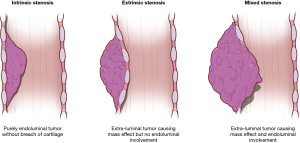
The most common indication for stenting is airway obstruction. This can present in three main fashions: intrinsic or endoluminal, extrinsic or extraluminal, or mixed (Figure 1). Purely intrinsic obstruction, typically due to malignancy, can often be managed with bronchoscopic interventions. However, stenting may still be required if significant tumor burden remains despite endoluminal therapies or if tumor regrows rapidly. Purely extrinsic obstruction due to compression by masses outside the airway require stenting because there is nothing to intervene upon within the airway lumen. A mixed presentation can also occur in which there is both intrinsic and extrinsic pathology. This often requires a combination of modalities to restore adequate patency to the affected airway and may requiring stenting if the extraluminal compression is found to be significant after the endoluminal component is cleared.
Another indication is weakness of the airway wall such as in tracheobronchomalacia in which the airway wall collapses to variable degrees. Defects in the airway wall such as airway fistulae (due to cancer or trauma, for example) or anastomotic dehiscence after lung resection or transplantation may also require a stent to bridge these gaps.
A much less common indication for stenting is when bleeding control with tamponade of an airway wall is needed. This has been typically done in the setting of diffuse airway wall bleeding such as due to tumor infiltration (4). Endoluminal ablative therapies can be used but a stent is a rapid intervention that can cover a wide area of disease relatively quickly.
Patient selection
Ultimately, the choice of whether to place an airway stent in a given patient depends on the risk/benefit ratio for the presenting clinical scenario. Several factors must be taken into consideration simultaneously.
Firstly, the exhibiting airway obstruction must be of sufficient severity as to explain the patient’s symptoms. Patients often have multiple potential reasons to have dyspnea and it may be that airway obstruction is a less likely culprit in a given case. For example, a longtime smoker presenting with mild airway obstruction from newly-diagnosed lung cancer is also at risk for and may concurrently have chronic obstructive pulmonary disease, pulmonary embolism, and/or cardiovascular disease. If the airway involvement in such an instance is not significant then one of these other factors (or a combination) may be the etiology for dyspnea in which case stenting will not be beneficial. It is generally held that central airway lumen must be reduced to approximately 50% of normal before a patient will develop symptoms (1). A tracheal diameter of 8 mm usually will lead to dyspnea on exertion. Reduction to 5 mm or less will lead to dyspnea at rest (1). In such cases, airway intervention should be considered, even if other contributing etiologies may also be at play.
Secondly, timing is a factor. In cases of severe obstruction, bronchoscopic intervention should be performed promptly. However, in certain cases where observation at the present time may be a reasonable plan (due to borderline severity of airway obstruction, for example), one must also consider whether delaying intervention will lead to a more challenging or risky procedure later. In those situations, it may be more prudent to intervene sooner rather than later.
Another consideration is whether bronchoscopy is the best intervention for a given case that clearly does require some form of intervention. In some instances, such as lymphoma or small cell lung cancer, for example, chemotherapy and/or radiation may improve airway obstruction relatively quickly and non-invasively. Any patient in extremis from severe airway obstruction requires bronchoscopy immediately, of course. Bronchoscopy and stenting likely would also be indicated in cases where a patient’s disease has been refractory to other therapies and a stent is the only remaining option to relieve symptoms. In cases of tracheobronchomalacia, surgery is the treatment of choice but stents are used as a trial intervention to ensure that restoration of airway lumen leads to symptomatic benefit (5). As such, this is a case where a stent is not the ideal choice but is a necessary temporary measure.
Finally, a few other factors are necessarily part of the risk/benefit comparison and these potential issues also need to be taken into account. Complications are not very common with airway stents but they do occur, some in the near-term but more in the longer-term (see section on ‘Complications’ below for more details). A given patient’s capacity to tolerate these complications (e.g., infection, stent migration) must be considered. From a sedation perspective, stents usually require bronchoscopy under general anesthesia and a patient’s ability to tolerate this needs to be incorporated in the decision-making process. Some patients with malignant central airway obstruction have a rather limited life expectancy and their goals of care need to be considered, including their willingness to undergo invasive procedures. It must be emphasized, however, that airways stents can certainly be palliative and helpful for relieving dyspnea even at the end of life (6). From a procedural technique perspective, there must be patent airways distal to the obstruction. Otherwise, stenting will be futile, opening part of an airway only to lead to obstruction distal to that. This may be ascertained by pre-procedural imaging but is not always clear until the time of direct visualization by bronchoscopy.
Importantly, aside from these important concepts, there are data to help guide the patient selection process. As part of the American College of Chest Physicians Quality Improvement Registry, Evaluation, and Education (AQuIRE) registry, Ost and colleagues collected and analyzed data from 1,115 bronchoscopies performed on 947 patients for malignant central airway obstruction at 15 different centers with 26 physicians (2). Central airway obstruction was defined as ≥50% occlusion of the trachea, mainstem bronchus, bronchus intermedius or lobar bronchus. There were statistical differences in practice patterns between the centers with regard to anesthesia, ventilation, rigid bronchoscopy, ablative techniques and stent use. Approximately one-half of patients had any endobronchial obstruction and nearly one-half had mixed obstruction; one in seven patients had any extrinsic obstruction. Over one-third of cases involved an airway stent for recanalization. In terms of outcomes, 93% of procedures were technically successful (defined as reopening the airway lumen to >50% of the normal diameter and connecting to a viable area of distal lung) and stent use was associated with higher rates of technical success. Notably, after therapeutic bronchoscopy, patients with worse baseline dyspnea (based on the Borg scale) had greater improvements in both dyspnea and health-related quality of life. Those with higher American Society of Anesthesiology score (an assessment of overall health) and poorer functional status had greater increases in health-related quality of life. As such, the patients that are often thought to be at greatest risk for these procedures may be the very ones that stand to gain the most from them. Additionally, patients with non-lobar obstruction also had greater improvements in both dyspnea and health-related quality of life. This is particularly relevant to the practice of stenting because stents are more-readily placed in larger airways than in lobar bronchi, there are more options commercially available for airway stents for these more central airways, and there is much more experience in the field of bronchoscopy with stenting in these locations as compared to lobar stents.
In all, several factors need to be taken into account when determining whether to intervene in a case of airway obstruction—and whether a stent is the best option therein. Other etiologies for a patient’s symptoms, timing, ability to tolerate complications, severity of symptoms and location of obstruction, among others, all must be synthesized and carefully weighed.
Stent selection
The ideal airway stent would have several characteristics (7). Firstly, it should be relatively easy to implant. It also must be able to be readily removed once it is no longer needed while simultaneously adhering to the airway well enough that it stays in place and does not migrate spontaneously. The diameter needs to be large enough to restore maximal patency but not so large as to possibly cause tissue ischemia. Preferably, flexibility will allow it to be placed into a narrowed airway and also accommodate physiologic airway movement (e.g., as occurs with coughing and respiration) yet also have sufficient radial force to counteract airway obstruction. Finally, it must also harmonize well enough with a given patient’s anatomy such that granulation tissue is minimized (see ‘Complications’ section below for further discussion) but not match the anatomy of a compressed airway so much that it simply replicates the obstruction. As such, the more personalized or customized a stent can be, the more likely it is to be a benefit to a patient rather than a detriment. Indeed, stents need to be as carefully selected as possible so as to not make a patient’s situation worse rather than better.
An assortment of airway stents are commercially available, usually categorized based on the material with which they are made—metal or silicone. They are sometimes further subcategorized based on shape and can have distinct advantages or disadvantages depending on the clinical indication (Table 1). Generally speaking, metal stents are easier to place than silicone stents and can be done via flexible bronchoscopy alone (without requiring rigid bronchoscopy). They also can mold or conform to a given airway fairly well and tend to migrate less often than silicone stents due to their design. Silicone stents are easier to remove, can be customized to a degree at the time of procedure, and are less costly than metal stents. Below we will review a representative sample of the available and most recent data on the use of airway stents for central airway obstruction.
Table 1
| Dumon Silicone (A) | Aero self-expandable metal fully covered (B) | Ultraflex self-expandable metal partially covered (C) | |
|---|---|---|---|
| Advantages | • Easy to remove | • Easier to place (no rigid bronchoscopy) | • Easier to place (no rigid bronchoscopy) |
| • Available in Y, tube, and hourglass shapes | • Can adapt to irregular anatomy | • Can adapt to irregular anatomy | |
| • Customizable at procedure time | • No risk of tumor ingrowth since fully covered | • Uncovered ends allow ventilation and may help prevent migration | |
| Disadvantages | • Requires rigid bronchoscopy | • Possible increased risk of infection | • Uncovered ends may allow tumor ingrowth and make removal more difficult |
| • Increased risk of granulation | • Lack of uncovered portion does not allow ventilation | ||
| Stents | 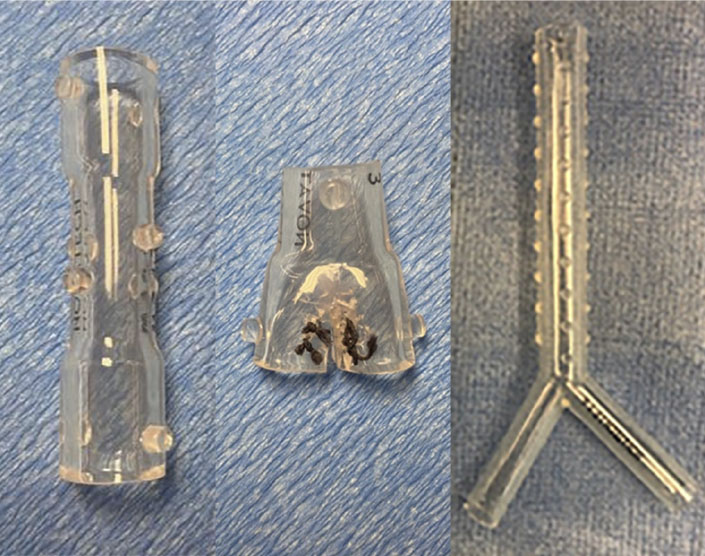 |
 |
 |
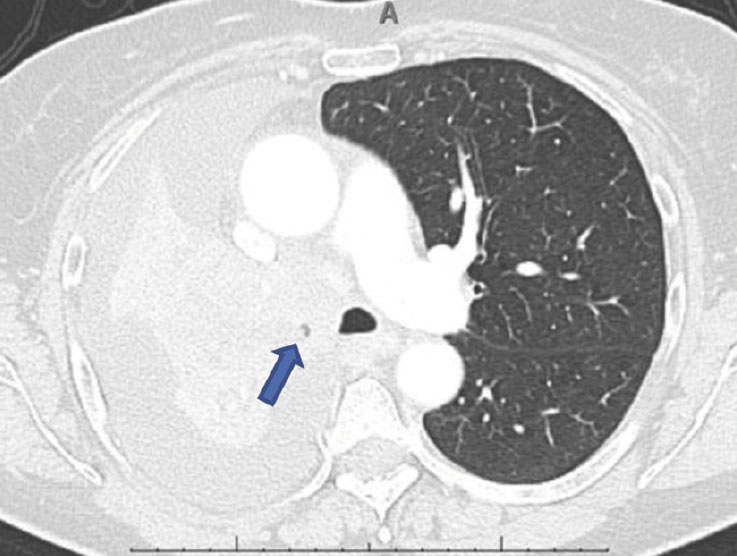
| Pre-intervention CT | 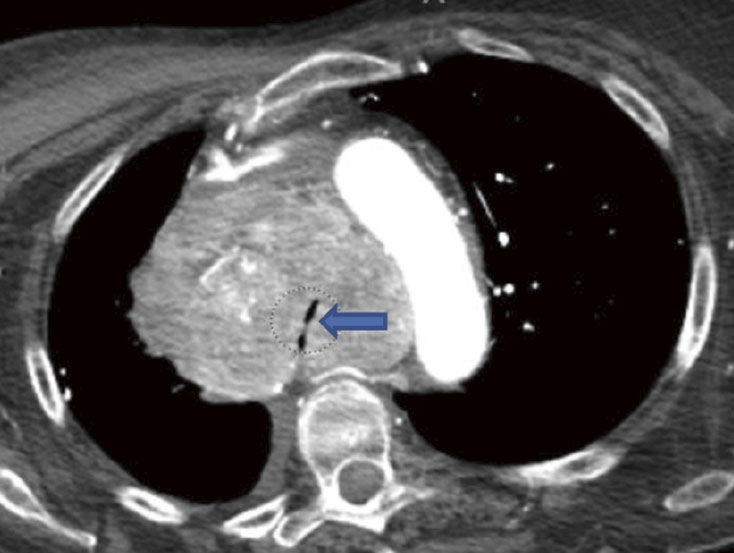 |
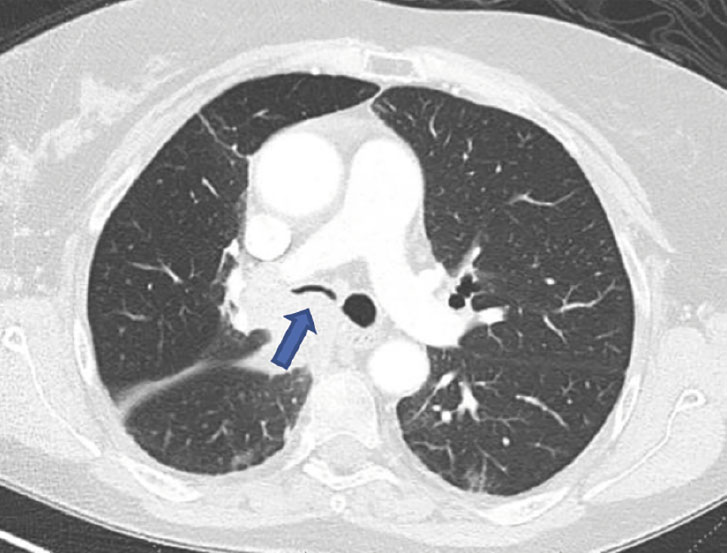 |
 |
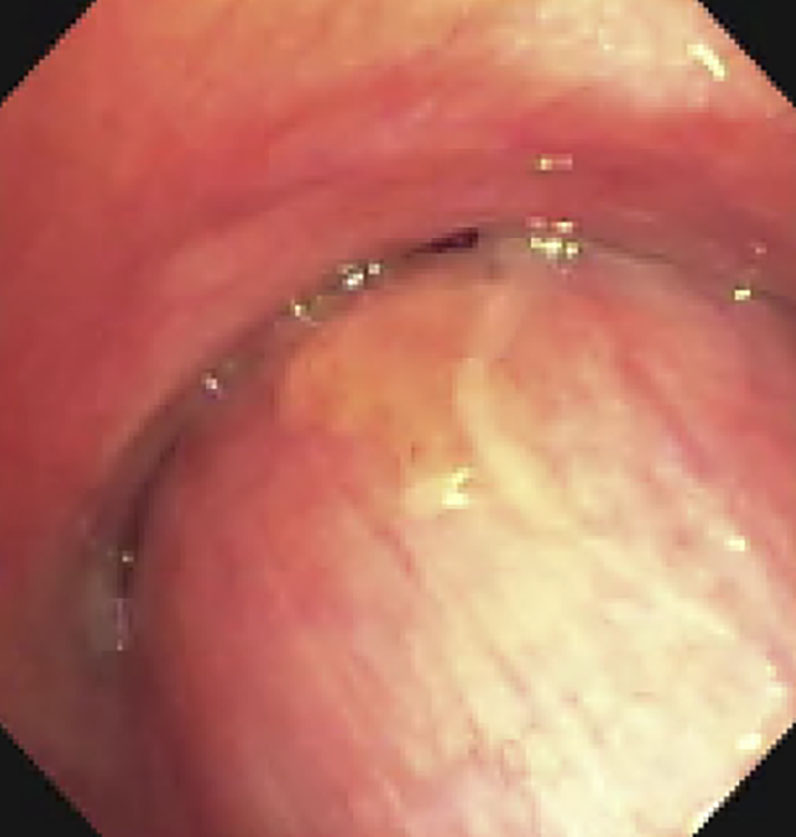
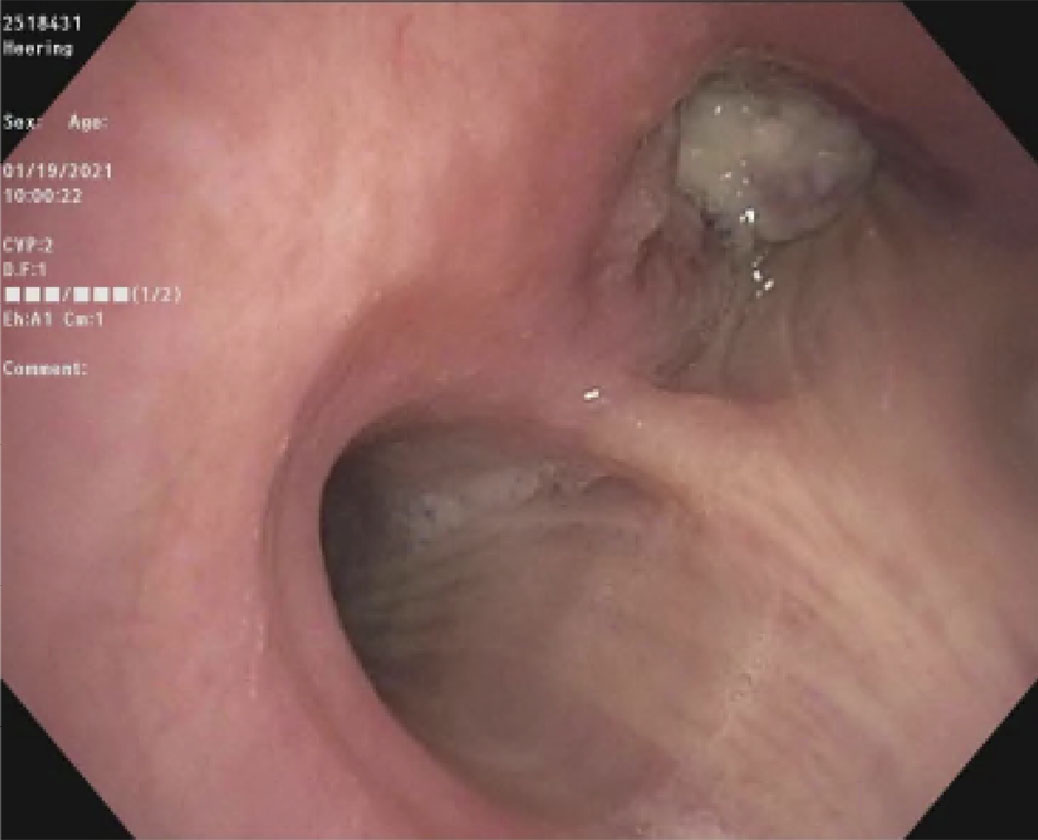
| Pre-intervention bronchoscopy | 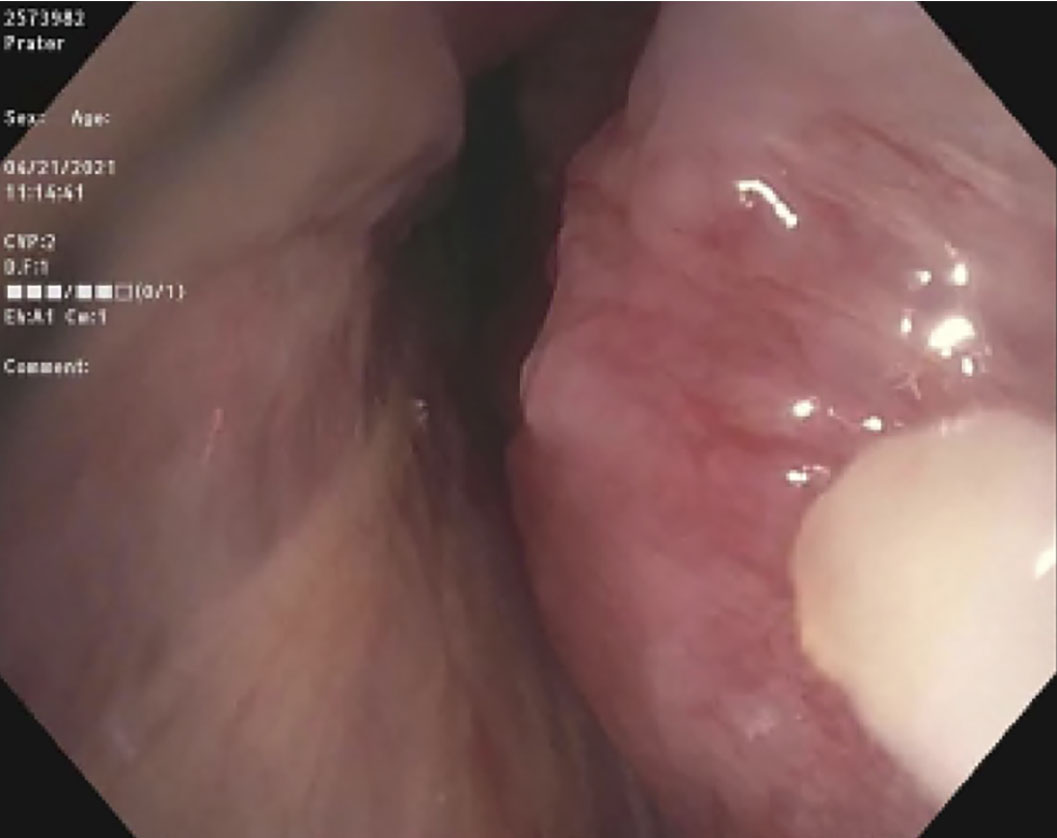 |
 |
 |
| Proximal end of stent | 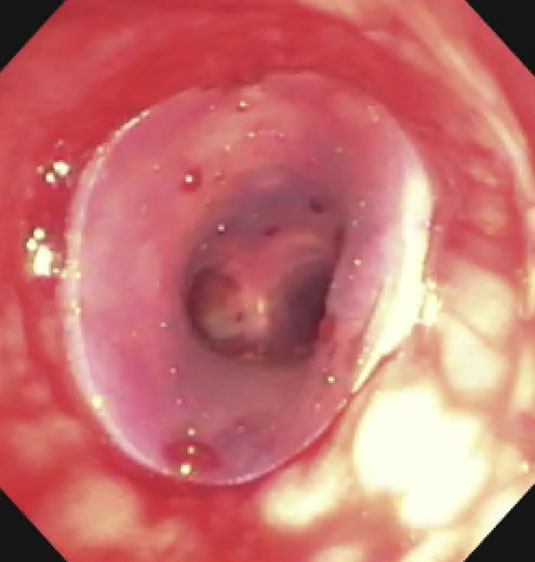 |
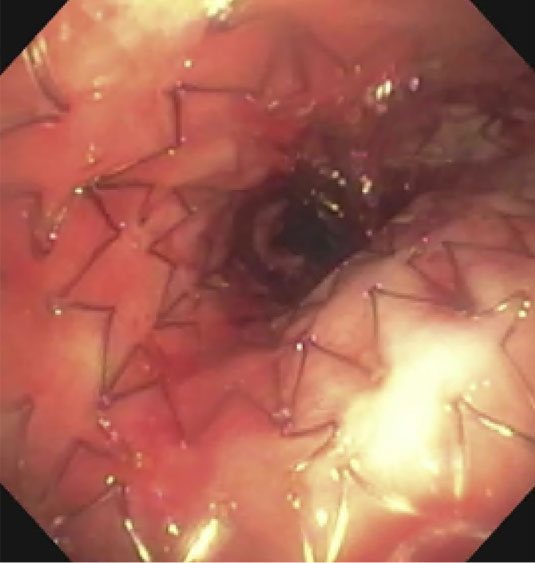 |
 |
| Distal end of stent | 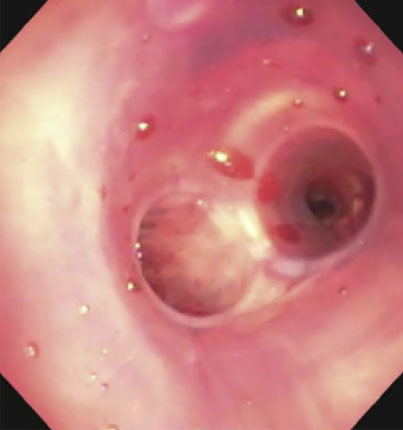 |
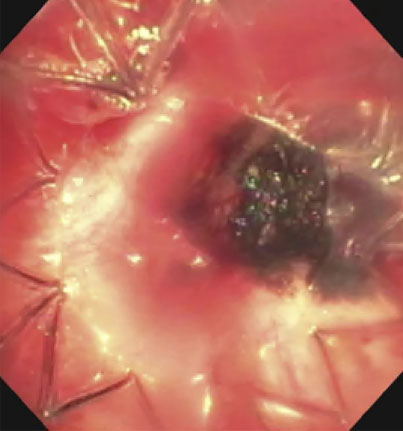 |
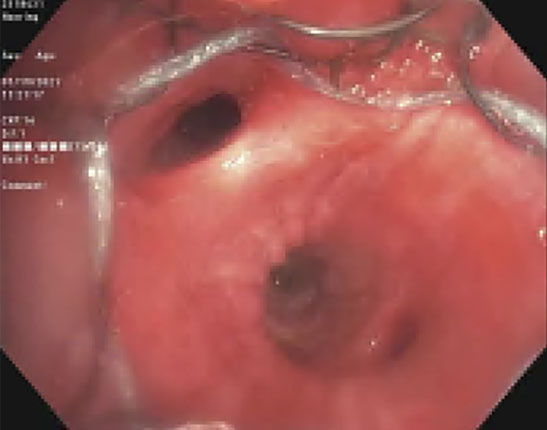 |
(A): Silicone Y stent placed to relieve critical tracheal obstruction. The trachea is reduced to a narrow slit (arrow). Different silicone stents are shown: hourglass, hourglass customized into mini-Y, and Y stent. (B): Aero stent used to treat severe extrinsic obstruction (arrow) of the right main stem bronchus from a mass compressing the posterior membrane of the airway. (C): Ultraflex stent used to relieve obstruction of the right main stem bronchus which has been compressed down to a pinhole (arrow). There is resultant complete post-obstructive collapse of the right lung. CT, computed tomography.
Metal stents
Metallic stents were initially developed approximately four decades ago and gained favorable attention for some time. However, possibly because use outpaced knowledge of how to prevent and manage complications, a prevalence of poor outcomes were observed, and the Food and Drug Administration (FDA) administered a black box warning for the use of metallic stents in benign airway disease (8). As a result, a decline in the practice resulted for some time. The history surrounding this as well as the evolution of subsequent generations of stents has been reviewed extensively by Avasarala (9). Indeed, as stent technology has advanced over the years with progressive improvements in their design, there has been a resurgence in their application over the last several years.
The original metallic stents were made of stainless steel or tantalum. More modern stents are made of nitinol, a metal alloy of nickel and titanium. Initially developed by the military in the late 1950s, it has since found a variety of applications including in electronics, civil engineering, and even the automobile industry (10). Nitinol has two important properties that make it particularly useful for recanalization of obstructed airways. Shape memory allows it to take on a deformed shape below a certain temperature and even to retain that shape when the external force is removed (10). However, once it is heated above its ‘transformation temperature’, it returns to its original, undeformed conformation (hence the term, ‘self-expandable metallic stent’ or SEMS). This allows stents to be compressed and placed into a deployment system that can be inserted into a narrowed airway and then expand to the intended size at body temperature once deployed. Secondly, superelasticity allows the stent to withstand significant amounts of strain (e.g., from coughing) as well as to conform to the shape of a given stenosis (11,12).
Metal stents now are typically produced either covered by a membrane or uncovered. The latter are completely porous, akin to a mesh or chain-linked fence. As such, they are not appropriate for malignant endobronchial obstruction in which case surrounding tumor would simply grow into the stent. But these can be successfully used for anastomotic dehiscence after lung transplantation, for example, as the formation of granulation tissue along the uncovered stent can help seal the defect. Covered stents typically employ polyurethane or silicone membranes. These membranes cover the stent and thereby prevent tumor in-growth. A disadvantage of this is that mucociliary function underneath the covering is compromised and also, in the case where an obstruction traverses a side-branch airway, that airway will be obstructed by the stent. Metal stents that are covered with silicone have been termed ‘hybrid’ stents because they are composed of both metal and silicone. The Bona stent (Thoracent, Huntington, NY, USA), Aero (Merit Medical Endotek, South Jordan, UT, USA) and Ultraflex (Boston Scientific, Marlborough, MA, USA) stents are such hybrid stents and are also among the more popularly used.
There are several series that have been published over the years and a representative sample will be discussed here. A large series of 376 metallic stents was described by Sinha and colleagues (13). All were placed to manage post-transplant complications, mostly bronchial stenosis with a minority being for anastomotic dehiscence. Slightly over half of the cases employed the Bonastent, with the remainder using the Atrium iCast (Atrium Medical, Hudron, NH, USA) or Aero stents. Median individual stent duration was 22.5 days per stent with a median total of 176 stent-days per patient. Approximately half had to be removed due to mucus plugging with granulation and migration occurring in a minority of patients. Two cases of major bleeding occurred at stent removal, one leading to cardiac arrest and the other to pneumonectomy. There was no significant difference in complication rates between the different stent products except for a higher (albeit small, 6.5%) fracture rate with the Bonastent.
Holden recently published the largest series of cases of the Bonastent (14). Sixty stents were placed in 50 patients, 90% for malignancy. Most were placed in the bronchus intermedius or trachea, with a mean stent duration was 74 days. Seventy percent of patients had improvement in respiratory symptoms (dyspnea, cough, or stridor) within 30 days. The mean modified Medical Research Council (mMRC) dyspnea scale score decreased over time (lower numbers reflect less dyspnea). The overall complication rate was 54% at a mean follow-up of approximately 4 months, with a higher rate occurring after 30 days. Early complications (<30 days) included mucus plugging, granulation formation, and migration, all occurring in 8% or less of cases. Late complications (≥30 days) were the same but more frequent (25% or less). There were no intraoperative complications.
Ishida and colleagues similarly reported a recent series of patients treated with the Aero stent (15). Forty-two stents were placed in 36 patients; 5 stents were placed for tracheoesophageal fistula with the remainder for malignant airway obstruction. Of the patients that required oxygen prior to stent placement, 78% no longer did, including 5 patients who had been mechanically ventilated. There was also a statistically significant improvement in pulmonary function testing among those for whom this data was available. Stent-related complications occurred in one-third of patients, with 14% experiencing migration and 7% granulation.
Regarding the Ultraflex stent, Chung and colleagues published the largest series to date in 2011 (16). Over a period of approximately 6 years, 149 patients underwent stent placement (72 for benign disease and 77 with malignant). The main indications for those with benign disease were malacia and stenosis due to prior intubation or tuberculosis. The primary reasons for those with malignant disease were airway invasion by lung cancer and tracheoesophageal fistula. Symptoms improved in a higher proportion of patients with benign disease (77%) compared to those with malignant disease (52%). This may be attributed to the additional burden of symptoms and attendant comorbidities in patients with cancer. The overall complication rate was also significantly higher in patients with benign disease (42% vs. 21%) but this may be due to the fact that those with benign disease have a longer life expectancy which is also likely reflected in the much higher follow-up time (median, 429 days compared to 57 days). Granulation tissue formation was the most common complication for both groups of patients (19% for benign disease, 10.5% for malignant). The next most common complication was stent fracture for benign disease (16.4%) and stent migration for malignant disease (8.4%).
Also on the Ultraflex stent, Breitenbucher and colleagues published the next largest series in 2008 (17). Sixty-two stents were placed in 60 patients; 82% were covered. Technical success was achieved in all cases. In those for whom pulmonary function tests (PFTs) were available, the mean forced expiratory volume in 1 second (FEV1) significantly improved from 1.45 to 1.78 liters. Overall, complications were noted in approximately one-quarter of patients with mucus plugging (8%) being the most common. Granulation tissue developed in 5%. In most patients, these issues were managed bronchoscopically without requiring stent removal. Tumor restenosis and stent migration occurred in 5% of cases each. There was no stent-related mortality.
Silicone stents
Silicone stents are placed after folding in a loading device and inserting into a metal rod that fits through a rigid telescope. They are typically deployed distal to an area of obstruction and then pulled back into place with long rigid forceps. Unlike metallic stents, customization can be performed at the time of bronchoscopy. For example, they can be trimmed down to a desired length with scissors and side holes can be cut out to allow for aeration of airway branches that might otherwise become unintentionally blocked along the length of the stent.
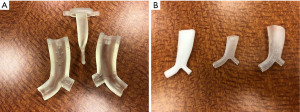
The first dedicated silicone tracheobronchial stent was described by Dumon in 1990 (18). This was a customized version of the previously used Montgomery T-tube used for tracheal stenosis and little has needed to change in terms of its design over the years. The Dumon stent (Novatech, La Ciotat, France) has been the standard but other silicone stents exist including the Polyflex (Boston Scientific), the Hood (Hood Laboratories, Pembroke, MA, USA), and the Noppen stents (Reyden Medical Supply, Lennik, Belgium). Silicone stents can be straight, Y-shaped (to stent across a narrowed carina) or hourglass-shaped (with a narrow central portion and wider diameters both ends). They are typically lined with studs that help anchor the stents to the airways but also allow a small space between the airway and the stent, allowing for the continued benefit of mucociliary function. Given their intrinsically-variable shape, the Y and hourglass stents, in particular, can be creatively modified to tailor to the needs of a given obstructed airway (19,20).
In terms of the available data, the largest case series remains the original report by Dumon himself nearly three decades ago (21). One thousand five hundred seventy-four stents were placed in 1,058 patients in four centers in three European countries over 7 years. Most were placed for malignant central airway obstruction. Benign indications included tracheal stenosis and post-surgical stenoses (i.e., post-lung resection or post-transplant). Overall, over half were placed into the trachea and nearly one-quarter in the left mainstem bronchus and slightly less in the right mainstem bronchus. The mean duration of stent placement for cicatricial tracheal stenosis was 1.2 years with the longest duration 6.2 years. For malignant disease, the mean duration was 4 months with the longest duration 4.7 years. Symptomatic outcomes were not described. Complication rates were relatively low with migration occurring in 9.5% of cases, granulation formation in 7.9%, and mucoid obstruction in 3.6%.
The same year as the Dumon study, Cavaliere and colleagues published their experience placing 393 silicone stents in 306 patients as part of a larger series describing therapeutic bronchoscopy for malignant airway obstruction (22). Over one-third were placed in the trachea with an even distribution of stents placed in the right lung, left lung, or a combination. All patients experienced improvement in pulmonary function tests and quality of life (though more specific data were not reported). The median survival of patients with a stent in place was 108 days with a maximum of 1,720 days.
Wood and colleagues described their experience placing 309 stents in 143 patients, 87% of which were silicone (23). Two-thirds of the indications were for malignant disease. Approximately one-quarter had disease primarily involving the trachea, for example, but half had disease involving multiple airways. Notably, 95% of patients experienced significant symptomatic improvement. Regarding complications, 36% had partial obstruction by secretions or granulation and 5% of stents experienced migration.
Dutau et al. published a series of 90 Dumon Y-stents placed in 86 patients who had presented with dyspnea, cough, and/or hemoptysis (24). All were placed for consequences of cancer, mostly airway obstruction though some were placed due to airway-esophageal fistula. Only two procedure-related adverse events were reported; one being a week-long cough post-stent and the other being migration of a stent which was subsequently removed without further untoward events. All patients tolerated their stent well and all reported improvement in symptoms, though no formal evaluation tool was utilized to measure this. Average stent duration in situ was 133 days with median time of survival post-stent being 181 days.
Silicone stents have also been used to treat benign airway obstructions. A recent meta-analysis, the first such study of silicone stents for this indication, evaluated 395 patients across 8 studies (25). Silicone stents showed a curative rate over 40%. This was defined as the proportion of patients who were able to have their stents removed without symptomatic restenosis in one year. The stability rate of the stents (proportion of patients who maintained stable stent placement) was similar. As a result, an “effective rate” was over 75%—the sum of the curative and stability rates. In terms of complications, the migration rate was 25% with a granulation rate of 15.7%. No evidence of publication bias was found.
Complications
As mentioned above, airway stents are sometimes met with unintended consequences. The literature has described these thoroughly including the studies mentioned above. In this section, we will put forth studies that specifically examined complications with a more direct and sophisticated analytical approach.
The largest study that specifically looked at complications of stents and therapeutic bronchoscopy came out of the AQuiRE registry (26). Out of over one thousand procedures performed in 947 patients at 15 centers, slightly more than one-third involved stent placement. Among stents placed, 2/3 were metal stents and 1/3 silicone. Overall, among the entire study cohort, only 3.9% of patients experienced a complication with 0.5% resulting in death. Among the eight centers with data on ≥25 cases, complication rates ranged from 0.9% to 11.7% and the proportion of cases in which a stent was placed ranged from 13% to 69%. On multivariate analysis, risk factors for increased complication rates were urgent and emergent procedures, American Society of Anesthesiologists (ASA) score >3, redo therapeutic bronchoscopy and use of moderate sedation. Stents were not associated with an increased risk of complications. However, also on multivariate analysis, risk factors for increased risk of death within 30 days of the procedure included Zubrod score >1, ASA score >3, any intrinsic or mixed obstruction, and stent placement. Y stents had a higher risk of death [odds ratio (OR) =4.92] than tube stents (OR =1.72). This latter association could be attributed to confounding by the presence of a greater burden of disease in patients requiring a stent or in those for whom all other therapeutic measures had been exhausted with a stent being the final alternative. In all, based on this study, stents are safe but cases should be selected carefully as we have suggested above.
Another instructive study specifically compared patients with and without stents with regard to stent-related infection risk (27). Seventy-two patients with malignant central airway obstruction were evaluated who underwent therapeutic bronchoscopy. Twenty-four of these received one or more stents. Overall, 23 of 72 (32%) developed lower respiratory tract infections (LRTI). A unique and useful aspect of this study was that incidence rates of infection were determined, not just incidence proportions as in most studies (incidence rates are more appropriate because they reflect events per person-time at risk). Also, by comparing stented and non-stented groups, the incremental risk of infection was elucidated. The incidence rate of LRTI in patients with stents was 0.0057 infections per person-day; the corresponding rate in patient without stents was 0.0011 infections per person-day. The resultant incidence rate risk difference of 0.0046 infections per person-day, in turn, translates into a 13% increased risk of LRTI per month. This is equal to one infection for every 8 stents placed. Twenty-six percent of patients with infections died within 2 weeks of stent placement. Indeed, after multivariate analysis, LRTI was associated with mortality. Taken together, this study showed that respiratory infections are increased after stent placement. And, given correlation (though not necessarily causation) with survival, this evidence strongly suggests very judicious use of stents.
Another informative study compared complications of various individual stents (28). Ost and colleagues used time-to-event analysis to evaluate 195 stents in 172 patients: Ultraflex in 60%, Aero in 16% and Dumon silicone stents (either tube or Y-shaped) in 24%. The most common complications were infection, migration, granulation tissue formation, and mucus plugging. Seventy-three patients developed 106 LRTI. The median time to infection was 1 month (range, 0–35 months). Over half of these were hospitalized and nearly one-quarter died within 2 weeks of their infection. On multivariate analysis, only the Aero stent was significantly correlated with infection. This particular outcome was instructive with respect to analytical approaches because the incidence proportion of stent infections was the same across the different stents. However, the incidence rate of stent infections was double for Aero compared to other stents. Regarding migration, this was analyzed only with regard to metal stents and silicone tube stents; because silicone Y stents rarely migrate, these were excluded. Median time to migration was 1.4 months (range, 0–36 months). On multivariate analysis, only silicone tube stents were correlated with this outcome. Regarding granulation tissue, the median time to event was also 1.4 months (range, 0–36 months). Interestingly, silicone stents and LRTI were significantly associated with granulation tissue formation. Mucus plugging occurred in nearly one-quarter of cases with a median time to event of 1.3 months (range, 0–46 months). In the final analysis, a left-sided stent and silicone stents were associated with an increased risk of mucus plugging. There was no statistical association between stent type and hemoptysis, tumor overgrowth, and stent fracture. In terms of overall survival, median follow-up time was 3 months (range, 0–73 months). One hundred forty-six patients of the 172 died. In the final multivariate model, silicone and tracheal stents were associated with decreased mortality but pre-stent radiation therapy and LRTI were associated with increased mortality. These are not necessarily causative relationships, of course, due to the risk of selection bias in this non-randomized study. Silicone stents, for example, will be placed in patients who are fit enough to tolerate rigid bronchoscopy. However, the link between LRTI and survival may indeed be real, as was also suggested by the study by Grosu and coworkers described above.
Overall, stents can certainly be beneficial in select cases but potential complications must be considered in the decision about whether to place one or not in a given case. Even when clearly indicated, however, these unintended consequences must be taken into account and the available data can help determine when/if to perform surveillance bronchoscopy, timing of follow-up, and patient education.
Future directions
Technological advancements regarding airway stents have taken several avenues. Research has been conducted in the effort to develop biodegradable stents that do not require removal but, rather, disintegrate over time. This has the benefit of avoiding a second bronchoscopy for stent removal and theoretically decreases complications since the stents do not remain in place for a prolonged period of time (29). Drug-eluting stents have also been explored, based on the idea that local release of certain agents can treat underlying tumor (cisplatin- or paclitaxel-eluting) or reduce complications (mitomycin-eluting), for example (30-32). These have been reviewed elsewhere (12). In this section, our focus will be on stents produced by three-dimensional (3D) printing as this is a more recent development.
Three-dimensional printing (3DP) is a versatile technology that has made significant inroads into clinical medicine. Applications have been found in various procedural and surgical disciplines, including those treating the nervous, craniofacial, cardiovascular, digestive, genitourinary, and musculoskeletal systems (33). Its introduction into interventional pulmonology is in its early stages but is an attractive investigative idea because stents could be produced that are designed to match a specific patient’s anatomy. Obstructed airways can be tortuous and irregular, yet, as described above, typical stents are of a single diameter and rather uniform in their shape. Modifications to attempt to tailor a stent to a given airway are limited: silicone stents can be cut shorter or have a hole cut for ventilation of an airway branch, for example. Y-stents can be ordered with specific angles, widths and lengths but these take weeks to produce and are still of a single diameter in a given limb. 3D printing, theoretically, could allow for a stent to be produced on-site, within hours, and specifically based on a given patient’s computed tomography (CT) scan (Figure 2).
Initial cases of clinical success with 3D-printed stents have been reported. In France, Guibert and colleagues published the first report of a 3D-printed mold that was made using software that integrated data from a patient’s CT scan. Medical grade silicone was poured into this mold to produce a stent that was then placed bronchoscopically to relieve bronchial stenosis in a post-lung transplant patient (7). Immediately after stent placement, the patient experienced durable improvements in dyspnea, quality of life, and objective functional parameters. Soon after this initial report, the same group published a series of 10 such cases of ‘anatomically complex airway stenosis’, defined as cases in which the stenosis was too complex to allow for a commercially-available stent or in which such a stent had previously failed (34). All stents were implanted without complication via rigid bronchoscopy. At 3 months, there was one case of mucus plugging, two cases of migration, and one case of significant cough; three required removal. Nine of 10 cases were felt to have great congruence between the stent and the airway. Eight of ten demonstrated improvements in dyspnea, quality of life, and pulmonary function testing. Contemporaneously, in the U.S., Gildea et al. also used 3D printing to treat the complex airway obstruction of two patients with granulomatosis with polyangiitis (formerly, Wegener’s granulomatosis). Both patients improved in a variety of measures as well (35). These stents were initially used under the FDA’s compassionate use program but have since received FDA approval (36). Finally, Schweiger et al. also recently reported the use of stents produced in a similar fashion to bring relief to two patients with severe tracheobronchomalacia in Austria (37).
Conclusions
Airway stents are clearly a technology that has been refined over the years. The application of stents must follow a careful cogitation of multiple clinical factors in order to select the proper patient and proper stent. As the field of interventional pulmonology has progressed over time and as research has advanced, we now have the experience and the data to help guide these decisions. Future directions should include the continued development of stents that reduce complications while maintaining their effectiveness.
Acknowledgments
We thank David Aten, MA, CMI from the Biomedical Visualization Department of MD Anderson Cancer Center for help with medical illustration for this manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Mediastinum for the series “Management of Airway and Vascular Invasion in the Mediastinum”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-22-65/coif). The series “Management of Airway and Vascular Invasion in the Mediastinum” was commissioned by the editorial office without any funding or sponsorship. BFS served as the unpaid Guest Editor of the series. RFC served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Mediastinum from April 2022 to March 2024. RFC reports research grants from Siemens and Intuitive and he is a paid consultant for Siemens, Intuitive and Johnson and Johnson. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Michaud G. Malignant central airway obstruction. In: Ernst A, Herth F. editors. Principles and Practice of Interventional Pulmonology. New York, NY, USA: Springer, 2013.
- Ost DE, Ernst A, Grosu HB, et al. Therapeutic bronchoscopy for malignant central airway obstruction: success rates and impact on dyspnea and quality of life. Chest 2015;147:1282-98. [Crossref] [PubMed]
- Murgu SD, Egressy K, Laxmanan B, et al. Central Airway Obstruction: Benign Strictures, Tracheobronchomalacia, and Malignancy-related Obstruction. Chest 2016;150:426-41. [Crossref] [PubMed]
- Sivaramakrishnan P, Mishra M, Sindhwani G, et al. Novel use of metallic stent to control post-debulking bleeding in a patient with central airway obstruction. BMJ Case Rep 2022;15:e252848. [Crossref] [PubMed]
- Kheir F, Majid A. Tracheobronchomalacia and Excessive Dynamic Airway Collapse: Medical and Surgical Treatment. Semin Respir Crit Care Med 2018;39:667-73. [Crossref] [PubMed]
- Vonk-Noordegraaf A, Postmus PE, Sutedja TG. Tracheobronchial stenting in the terminal care of cancer patients with central airways obstruction. Chest 2001;120:1811-4. [Crossref] [PubMed]
- Guibert N, Didier A, Moreno B, et al. Treatment of Post-transplant Complex Airway Stenosis with a Three-Dimensional, Computer-assisted Customized Airway Stent. Am J Respir Crit Care Med 2017;195:e31-3. [Crossref] [PubMed]
- Lund ME, Force S. Airway stenting for patients with benign airway disease and the Food and Drug Administration advisory: a call for restraint. Chest 2007;132:1107-8. [Crossref] [PubMed]
- Avasarala SK, Freitag L, Mehta AC. Metallic Endobronchial Stents: A Contemporary Resurrection. Chest 2019;155:1246-59. [Crossref] [PubMed]
- Nickel titanium. Available online: https://en.wikipedia.org/wiki/Nickel_titanium#Applications
- Shayesteh Moghaddam N, Saedi S, Amerinatanzi A, et al. Achieving superelasticity in additively manufactured NiTi in compression without post-process heat treatment. Sci Rep 2019;9:41. [Crossref] [PubMed]
- Sabath BF, Ost DE. Update on airway stents. Curr Opin Pulm Med 2018;24:343-9. [Crossref] [PubMed]
- Sinha T, Ho TA, van der Rijst N, et al. Safety of hybrid bronchial stents in transplant airway complications: a single center experience. J Thorac Dis 2022;14:2071-8. [Crossref] [PubMed]
- Holden VK, Ospina-Delgado D, Chee A, et al. Safety and Efficacy of the Tracheobronchial Bonastent: A Single-Center Case Series. Respiration 2020;99:353-9. [Crossref] [PubMed]
- Ishida A, Oki M, Saka H. Fully covered self-expandable metallic stents for malignant airway disorders. Respir Investig 2019;57:49-53. [Crossref] [PubMed]
- Chung FT, Chen HC, Chou CL, et al. An outcome analysis of self-expandable metallic stents in central airway obstruction: a cohort study. J Cardiothorac Surg 2011;6:46. [Crossref] [PubMed]
- Breitenbücher A, Chhajed PN, Brutsche MH, et al. Long-term follow-up and survival after Ultraflex stent insertion in the management of complex malignant airway stenoses. Respiration 2008;75:443-9. [Crossref] [PubMed]
- Dumon JF. A dedicated tracheobronchial stent. Chest 1990;97:328-32. [Crossref] [PubMed]
- Schwalk AJ, Marcoux M, Swisher SG, et al. Development of Miniature Y Stent for Treatment of Postoperative Bronchial Stenosis. Ann Thorac Surg 2020;110:e99-101. [Crossref] [PubMed]
- Sabath B, Casal RF. The (Hour)glass Half-Full: Modified Silicone Hourglass Stents for the Treatment of Central Airway Obstruction. Cureus 2021;13:e15501. [Crossref] [PubMed]
- Dumon JF, Cavaliere S, Díaz-Jimenez JP, et al. Seven-Year Experience with the Dumon Prosthesis. Journal of Bronchology 1996;3:6-10. [Crossref]
- Cavaliere S, Venuta F, Foccoli P, et al. Endoscopic treatment of malignant airway obstructions in 2,008 patients. Chest 1996;110:1536-42. [Crossref] [PubMed]
- Wood DE, Liu YH, Vallières E, et al. Airway stenting for malignant and benign tracheobronchial stenosis. Ann Thorac Surg 2003;76:167-72; discussion 173-4. [Crossref] [PubMed]
- Dutau H, Toutblanc B, Lamb C, et al. Use of the Dumon Y-stent in the management of malignant disease involving the carina: a retrospective review of 86 patients. Chest 2004;126:951-8. [Crossref] [PubMed]
- Chen DF, Chen Y, Zhong CH, et al. Long-term efficacy and safety of the Dumon stent for benign tracheal stenosis: a meta-analysis. J Thorac Dis 2021;13:82-91. [Crossref] [PubMed]
- Ost DE, Ernst A, Grosu HB, et al. Complications Following Therapeutic Bronchoscopy for Malignant Central Airway Obstruction: Results of the AQuIRE Registry. Chest 2015;148:450-71. [Crossref] [PubMed]
- Grosu HB, Eapen GA, Morice RC, et al. Stents are associated with increased risk of respiratory infections in patients undergoing airway interventions for malignant airways disease. Chest 2013;144:441-9. [Crossref] [PubMed]
- Ost DE, Shah AM, Lei X, et al. Respiratory infections increase the risk of granulation tissue formation following airway stenting in patients with malignant airway obstruction. Chest 2012;141:1473-81. [Crossref] [PubMed]
- Lischke R, Pozniak J, Vondrys D, et al. Novel biodegradable stents in the treatment of bronchial stenosis after lung transplantation. Eur J Cardiothorac Surg 2011;40:619-24. [Crossref] [PubMed]
- Wang T, Zhang J, Wang J, et al. Paclitaxel Drug-eluting Tracheal Stent Could Reduce Granulation Tissue Formation in a Canine Model. Chin Med J (Engl) 2016;129:2708-13. [Crossref] [PubMed]
- Chao YK, Liu KS, Wang YC, et al. Biodegradable cisplatin-eluting tracheal stent for malignant airway obstruction: in vivo and in vitro studies. Chest 2013;144:193-9. [Crossref] [PubMed]
- Zhu GH, Ng AH, Venkatraman SS, et al. A novel bioabsorbable drug-eluting tracheal stent. Laryngoscope 2011;121:2234-9. [Crossref] [PubMed]
- Cornejo J, Cornejo-Aguilar JA, Vargas M, et al. Anatomical Engineering and 3D Printing for Surgery and Medical Devices: International Review and Future Exponential Innovations. Biomed Res Int 2022;2022:6797745. [Crossref] [PubMed]
- Guibert N, Didier A, Moreno B, et al. Treatment of complex airway stenoses using patient-specific 3D-engineered stents: a proof-of-concept study. Thorax 2019;74:810-3. [Crossref] [PubMed]
- Gildea TR, Young BP, Machuzak MS. Application of 3D Printing for Patient-Specific Silicone Stents: 1-Year Follow-Up on 2 Patients. Respiration 2018;96:488-94. [Crossref] [PubMed]
- Pacetti A. FDA Approves 3D-printed Airway Stents Developed by Cleveland Clinic Doctor. 2020. Available online: https://newsroom.clevelandclinic.org/2020/01/08/fda-approves-3d-printed-airway-stents-developed-by-cleveland-clinic-doctor/
- Schweiger T, Gildea TR, Prosch H, et al. Patient-specific, 3-dimensionally engineered silicone Y-stents in tracheobronchomalacia: Clinical experience with a novel type of airway stent. J Thorac Cardiovasc Surg 2018;156:2019-21. [Crossref] [PubMed]
Cite this article as: Sabath BF, Casal RF. Airway stenting for central airway obstruction: a review. Mediastinum 2023;7:18.


