
Spindle cell thymoma and its histological mimickers
Introduction
Mediastinal tumors are uncommon neoplasms that can be derived from virtually any cell lineage, including tumors composed of spindle cells. The frequency of individual types of spindle cell tumors depends to a large degree on the location within the mediastinum; for instance, neurogenic tumors are most commonly found in the posterior mediastinum, while those of thymic or germ cell origin are typically restricted to the anterior mediastinum (1). In the anterior mediastinal compartment, the most common type of spindle cell neoplasm is spindle cell thymoma, a low-grade malignant epithelial tumor of the thymic gland. Spindle cell thymomas are known for their morphologic heterogeneity which can lead to difficulties in the diagnostic process, especially in small biopsy material (2). This histologic diversity is often the reason why other spindle cell tumors are considered in the differential diagnosis. In the anterior mediastinum, tumors with spindle cell morphology that can easily be mistaken with spindle cell thymoma include other epithelial-derived neoplasms, namely spindle cell carcinoid tumor and sarcomatoid thymic carcinoma as well as mesenchymal tumors, primarily solitary fibrous tumor (SFT), synovial sarcoma, and dedifferentiated liposarcoma or tumors with spindle cell morphology of various lineage that are either rare or are more commonly found in other mediastinal compartments. In this review, the differential diagnosis of spindle cell tumors of the anterior mediastinum will be discussed with emphasis on the clinical, histopathological, immunohistochemical and molecular characteristics of these rare neoplasms.
Spindle cell thymoma
Spindle cell thymomas, corresponding to type A thymomas in the World Health Organization classification, are the most common spindle cell tumors of the anterior mediastinum (3). These tumors predominantly affect adult patients in the 5th to 7th decade of life and are typically detected either due to symptoms of mass effect on adjacent anatomic structures, such as chest pain or shortness of breath, or as a result of imaging studies performed for unrelated reasons. Male and female patients are affected equally (2).
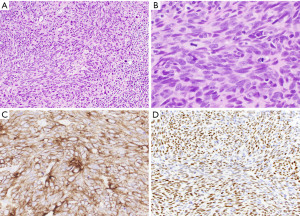
Grossly, spindle cell thymomas can be encapsulated, well-circumscribed or infiltrative masses with a firm tan cut surface showing vague lobulation. Cystic changes, hemorrhagic areas and even necrosis may be identified (2). The low power microscopic appearance of the classic type of these tumors is characterized by tumor cells arranged in sheets or storiform patterns that may be divided into lobules by discrete fibrous bands (Figure 1A,1B). Individual tumor cells have spindle-shaped nuclei with dispersed chromatin, indistinct nucleoli, and pale eosinophilic cytoplasm. The cytologic features are bland, with no significant nuclear pleomorphism and low or absent mitotic activity. Among these tumor cells, a small number of immature lymphocytes (thymocytes) is dispersed (Figure 1C). Perivascular spaces, empty spaces around blood vessels containing proteinaceous fluid and scattered lymphocytes, may be identified but are less often seen than in other types of thymoma (Figure 1D). Hassall corpuscles are typically absent. Spindle cell thymomas are notorious for displaying a wide morphological spectrum and familiarity with the different growth patterns is essential in order to avoid misdiagnosis (Table 1, Figure 2A-2H) (2).
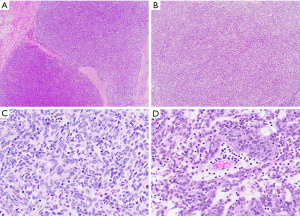
Table 1
| Morphologic subtype | Main histological findings |
|---|---|
| Classic spindle cell thymoma | Fascicular or storiform patterns; bland spindled/ovoid cells; scant lymphocytes |
| Micronodular spindle cell thymoma with lymphoid hyperplasia | Multiple tumor nodules composed of bland spindled/ovoid cells; embedded in lymphoid stroma with lymphoid follicles and germinal centers |
| Adenomatoid spindle cell thymoma | Short spindle-shaped tumor cells arranged in an adenomatoid/microcystic pattern; scant lymphocytes |
| Ancient (sclerosing) thymoma | Hypocellular spindle cell proliferation distributed in a prominent sclerotic stroma (>85%); calcification; cholesterol clefts; scant lymphocytes |
| Angiomatoid spindle cell thymoma | Spindle-shaped tumor cells separated by large, cavernous blood-filled spaces; scant lymphocytes |
| Desmoplastic spindle cell thymoma | Spindle-shaped tumor cells embedded in abundant hyalinized/fibroblastic stroma; scant lymphocytes |
| Spindle cell thymoma with papillary/pseudopapillary features | Spindle-shaped tumor cells arranged in papillary/pseudopapillary patterns; scant lymphocytes |
| Spindle cell thymoma with neuroendocrine pattern | Spindle-shaped tumor cells arranged in organoid nests, ribbons, or rosettes; scant lymphocytes |
| Spindle cell thymoma with neural pattern | Spindle-shaped tumor cells arranged in whorls (meningothelial pattern) or in alternating hypo- and hypercellular areas with perivascular hyalinization (schwannoma pattern); scant lymphocytes |
| Spindle cell thymoma with hemangiopericytoma pattern | Spindle-shaped tumor cells with prominent staghorn-like vasculature; scant lymphocytes |
| Spindle cell thymoma with cystic change | Spindle-shaped tumor cells separated by large cystic/pseudocystic spaces; reactive changes (chronic inflammation, fibrosis, cholesterol clefts, granulomas, etc.) |
| Atypical (type A) spindle cell thymoma | Spindle-shaped tumor cells with nuclear atypia, increased mitotic activity and foci of necrosis |
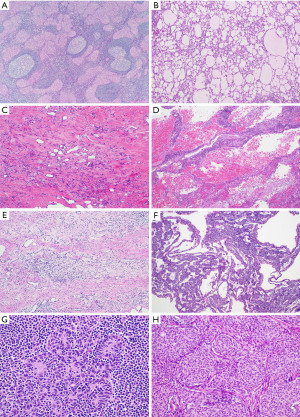
Rare cases of spindle cell thymoma showing mild to moderate nuclear atypia, increased mitotic activity and foci of necrosis but no anaplasia or atypical mitotic figures have recently been assigned as atypical type A thymomas (4). Their clinical significance, however, remains uncertain as they are not associated with more aggressive behavior than conventional spindle cell thymomas and tumor metastasis can occur also in cases devoid of any atypical histologic features (5,6).
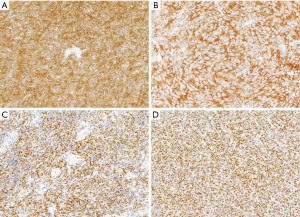
The immunohistochemical phenotype of spindle cell thymomas reflects its epithelial origin and is characterized by expression of a range of cytokeratins (AE1/AE3, CAM5.2, CK5/6) as well as p63/p40 (Figure 3A-3C) (7). Pax8 is another marker commonly positive in the tumor cells if the polyclonal antibody is used (Figure 3D) (8). Recently, a characteristic GTF2I mutation was identified in up to 80% of these tumors (9); whilst this is an important new discovery, its use for diagnostic purposes is currently limited as the gene is not yet included in commercial or academic next generation sequencing panels.
Despite their low-grade histopathologic features, spindle cell thymomas are malignant tumors and their prognosis is mostly dependent on the extent of disease at the time of diagnosis. With multimodal therapy, including surgery, chemotherapy and radiotherapy, the 5- and 10-year overall survival rates currently lie in the range of 80–100% (Table 2) (3).
Table 2
| Tumor type | Clinical | Histology | IHC | Molecular | Prognosis |
|---|---|---|---|---|---|
| Spindle cell thymoma | 5th–7th decade; M = F |
Heterogeneous; classic type with storiform or diffuse growth; bland spindle/oval cells; scant lymphocytes | CK+, CK5/6+, p63/p40+, Pax8 (polyclonal)+ | GTF2I mutation | Good |
| Spindle cell carcinoid tumor | 5th decade; M > F | Monotonous spindle cells arranged in organoid patterns | CK+, synaptophysin+, chromogranin+, CD56+ | – | Poor |
| Sarcomatoid thymic carcinoma | 6th decade; M > F | Solid or nested proliferation of malignant spindle cells; dense collagenous stroma; ± areas of spindle cell thymoma | CK+, CK5/6+, CK7+, p63/p40+, Pax8 (polyclonal)+, CD5+/−, c-kit+/− | – | Poor |
| SFT | 6th decade; M = F | Bland spindle cells in patternless pattern; keloid-like stroma; staghorn-like vasculature | CD34+, STAT6+, CK− | NAB2::STAT6 fusion | Good |
| Synovial sarcoma | 4th decade; M > F | Cellular proliferation of monotonous spindle cells; high mitotic activity; inconspicuous stroma; mast cells; staghorn-like vasculature | TLE1+, SS18-SSX+/SS18_CT+, CK+/− | SYT::SSX fusion | Poor |
| Dedifferentiated liposarcoma | 5th–6th decade; M = F |
Areas of well differentiated liposarcoma and non-lipogenic sarcoma (spindle cell proliferation with variable mitotic activity, nuclear pleomorphism) | MDM2+ | MDM2 amplification | Poor |
| Angiosarcoma | 3rd–4th decade; M = F |
Spindle cell proliferation with variable degrees of vasoformation; increased mitotic activity; necrosis | CD31+, CD34+, ERG+, CK+/− | – | Poor |
| Schwannoma | 4th–6th decade; M = F |
Bland spindle cells with wavy appearance; Antoni A and B areas; hyalinized blood vessels; lymphoid aggregates | S100+, SOX10+ | – | Good |
| Inflammatory myofibroblastic tumor | Children/young adults; M = F | Spindle cells with indistinct cell borders; fascicular growth pattern; mixed chronic inflammatory cell infiltrate; low mitotic activity | SMA+/−; ALK+/− | ALK gene rearrangement | Good |
| Sarcomatoid mesothelioma | Older adults; M > F |
Spindle cells with nuclear atypia, prominent nucleoli, increased mitotic activity | CK+; other mesothelioma markers variable | – | Poor |
| Intimal sarcoma | 5th decade; F > M |
Spindle cells with varying degrees of atypia and cellularity; subset with distinct differentiation; heterologous elements possible | Variable | MDM2 amplification | Poor |
SFT, solitary fibrous tumor; M, male; F, female; IHC, immunohistochemistry; CK, cytokeratin; ERG, erythroblast transformation specific related gene; SMA, smooth muscle actin; ALK, anaplastic lymphoma kinase.
Spindle cell carcinoid tumor of the thymus
Spindle cell carcinoid tumors of the thymus are rare tumors of the anterior mediastinum. Like spindle cell thymomas, these tumors originate in the thymic gland and are characterized by a proliferation of monomorphic spindle cells. They are more commonly seen in male patients with a peak incidence in the 5th decade of life (10-12). Approximately 25% of these neoplasms arise in patients with multiple endocrine neoplasia (MEN)-1 syndrome; 50% are functionally active and can induce Cushing syndrome (13). While a subset of patients remains asymptomatic, the majority of patients presents with various symptoms, including chest pain, weight loss, shortness of breath or superior vena cava syndrome (10). Thymic carcinoid tumors are unencapsulated neoplasms that may be well circumscribed or widely infiltrative. The cut surface is firm, gray and homogeneous and typically lacks the characteristic lobulation seen in thymoma. Gross areas of hemorrhage and necrosis are not usually identified (10,14,15). At the microscopic level, these lesions are characterized by an organoid growth pattern consisting of nests, ribbons, cords or rosette-like structures surrounded by delicate fibrovascular septa (Figure 4A). The tumor cells are monomorphic, small to medium-sized cells with moderate amounts of cytoplasm, pale eosinophilic cytoplasm, fine chromatin and inconspicuous nucleoli. In contrast to classic thymic carcinoid tumors, in the spindle cell variant, tumor cells have a fusiform shape and are often arranged in a hemangiopericytic growth pattern (Figure 4B) (16,17). Cytologic atypia is absent or mild and the mitotic activity is low (up to 3 per 10 high power fields). Necrosis, if present, is minimal (punctuate) (10).
The immunophenotype of these tumors is characterized by reactivity for cytokeratin and various markers associated with neuroendocrine differentiation, including synaptophysin, chromogranin A and CD56 (Figure 4C,4D) (10,18). The molecular characteristics of thymic neuroendocrine neoplasms are poorly understood and no diagnostic or clinically actionable mutations have been identified (19).
Owing to its location, the prognosis for thymic carcinoid tumors is often poor due to high tumor stage at the time of diagnosis. As a result, the overall survival rate at 5 years is around 50% which is significantly worse than that for spindle cell thymoma (10,20). For this reason, separation of the two entities becomes very important. While both tumors occur in the adult population, thymic carcinoid tumors are more commonly seen in male than in female patients. In addition, these tumors not uncommonly elicit paraneoplastic syndromes. While grossly, both tumors can show similar features, at a histologic level, spindle cell carcinoids typically lack fibrous bands, perivascular spaces and the lymphoid infiltrate that is so characteristic for spindle cell thymomas. On the other hand, spindle cell thymomas may show striking neuroendocrine morphology and immunohistochemical workup becomes indispensable in this setting. By immunohistochemistry, both tumors will express pancytokeratin but while spindle cell thymoma is positive for p63/p40 and negative for neuroendocrine markers the opposite is true for spindle cell carcinoid tumor (Table 2).
Sarcomatoid thymic carcinoma
Thymic carcinomas composed of spindle-shaped tumor cells are known as spindle cell or sarcomatoid thymic carcinomas. These tumors are epithelial-derived high-grade malignant tumors of the thymic gland; their spindle-shaped nature and origin in the anterior mediastinum is the reason why these tumors must be included in the differential diagnosis of spindle cell thymoma. Sarcomatoid thymic carcinomas occur in older adults with a slight male predominance (21). Chest pain, dyspnea and cough are the most common clinical symptoms.
Gross examination demonstrates well-circumscribed or locally infiltrative tumors with a firm cut surface and with foci of hemorrhage, necrosis and variable cystic change (21). Microscopically, these tumors commonly present as a solid proliferation of highly cellular spindle cells with fascicular or hemangiopericytic growth patterns and cytologically malignant features, including large, hyperchromatic nuclei with prominent nucleoli and frequent mitotic figures (3 to >10 mitoses per 10 high power fields) (Figure 5A,5B). The malignant cells are separated by dense collagenous stroma. Areas of hemorrhage, necrosis or cystic degeneration are frequently observed. In some cases, these tumors may display transition from spindle cell thymoma, indicating potential progression from a lower grade malignancy (21,22). Immunohistochemically, sarcomatoid thymic carcinomas are positive for cytokeratin as well as CK5/6, CK7, p63/p40 and polyclonal Pax8 (Figure 5C,5D) (8,22). Reactivity of these tumors for markers commonly positive in conventional thymic carcinomas, CD5 and CD117, is more variable in the sarcomatoid type (21,22). Characteristic molecular alterations that could be used for diagnostic or therapeutic purposes have not yet been identified. Contrary to spindle cell thymomas, sarcomatoid thymic carcinomas pursue an aggressive clinical course, with 50% of patients succumbing to the disease between 2 and 5 years after diagnosis according to the largest reported series (21).
The separation of sarcomatoid thymic carcinoma from spindle cell thymoma is primarily based on cytologic features with the former being characterized by nuclear pleomorphism, coarse nuclear chromatin, prominent nucleoli, and increased mitotic figures. Contrary to spindle cell thymomas, perivascular spaces and immature lymphocytes are not typically identified in sarcomatoid thymic carcinomas. Immunohistochemical workup is of limited use in this particular context as both neoplasms will show a similar immunophenotype (Table 2).
SFT
SFTs are mesenchymal tumors that most commonly arise in the visceral pleura and soft tissues but have also been recognized in the mediastinum, especially in the anterior and superior compartments (1). These tumors typically display low-grade spindle cell features and can occur in the vicinity of the thymic gland, thereby mimicking spindle cell thymomas. Mediastinal SFT are usually seen in the older population with a mean age in the 6th decade and without any specific sex predilection (23,24). Symptoms are related to mass effect of the tumor on neighboring organs and include chest pain, dyspnea and shortness of breath.
Grossly, mediastinal SFT are often large circumscribed but unencapsulated masses that can exceed 15 cm in size and show a tan-gray and firm cut surface (23,24). At microscopic examination, the low power appearance of the tumors is characterized by a “patternless pattern”, in which bland spindle-shaped tumor cells are arranged as randomly distributed hypo- and hypercellular areas (Figure 6A). Other growth patterns include fascicular, storiform, palisaded or herringbone patterns. Cytologic atypia is typically absent and mitotic activity is low. The stroma is heavily collagenized and often displays a keloid-like or carrot-shred quality (Figure 6B). Staghorn-like (hemangiopericytoma-like) vessels are commonly identified in these tumors (23,24). As all SFT are potentially malignant tumors, a four-criteria risk stratification model, which takes into account patient age, tumor size, mitotic activity and necrosis, is currently used to predict the risk of metastasis (25,26).
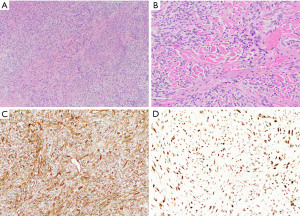
Immunohistochemically, the tumor cells of SFT show strong expression of CD34 and nuclear reactivity for STAT6 (Figure 6C,6D) (27-30). The latter is the result of a characteristic NAB2::STAT6 fusion transcript that has been identified in these tumors (31,32). Complete surgical resection is the treatment of choice for mediastinal SFT, both for primary tumors and recurrent disease (23,24,33-37).
SFTs can easily be mistaken for spindle cell thymomas, especially when displaying more cellular areas. Both tumors are composed of bland tumor cells, however, spindle cell thymomas will also contain a subtle lymphocytic infiltrate, in addition to delicate fibrous bands and perivascular spaces unlike SFT. In addition, immunohistochemical and molecular characteristics may be exploited to distinguish these two entities: while spindle cell thymomas will show strong expression of cytokeratin and p63/p40 and negative staining for STAT6, the opposite is true for SFT. On a molecular level, SFT harbor a NAB2::STAT6 fusion gene which can be used for diagnostic purposes (Table 2).
Synovial sarcoma
Synovial sarcoma is an aggressive spindle cell sarcoma that most commonly occurs in the soft tissue of young adults. In the thoracic cavity, most of these tumors involve the chest wall or lung, while the mediastinum is an exceptionally rare primary site accounting for <10% of all thoracic synovial sarcomas (38). Since most mediastinal synovial sarcomas arise in the anterior mediastinal compartment, these tumors may easily be mistaken for spindle cell thymomas. Most mediastinal synovial sarcomas occur in young adult patients with a mean age of 35 years and with a male predominance. Patients present with chest pain, shortness of breath, and pleural effusion or with constitutional symptoms, including fever, weight loss, and weakness (39).
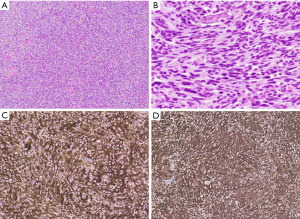
Grossly, mediastinal synovial sarcomas are large tumors (range, 5 to 20 cm). The cut surface shows gray-white to tan tumors with a soft, gelatinous or firm consistency and foci of hemorrhage and necrosis. Variable cystic changes may be observed. Microscopically, synovial sarcomas can display two major growth patterns, monophasic and biphasic types. Most mediastinal synovial sarcomas are of the monophasic type and consist of a monotonous spindle cell proliferation arranged in various growth patterns, including sheets, fascicles, storiform, hemangiopericytic or herringbone patterns (Figure 7A) (40). The tumor cells have oval to spindle-shaped nuclei, inconspicuous nucleoli and indistinct eosinophilic cytoplasm. Nuclear pleomorphism is not prominent but mitotic activity can be increased (2 to 12 mitoses/10 high power fields) (Figure 7B). The stroma is typically inconspicuous and the reason why the tumors appear highly cellular. After treatment, the stroma may become more prominent and consist of thick collagen or myxoid fibroconnective tissue (Figure 7C). Other characteristic findings in these tumors are the presence of vessels with a hemangiopericytoma-like or staghorn-like appearance, dystrophic calcification, scattered mast cells and cystic changes (39).
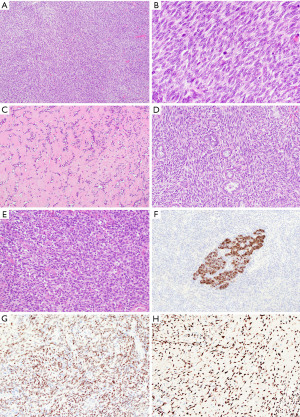
In the biphasic variant, the spindle cells are intermixed with glandular or pseudopapillary structures composed of round, cuboidal or columnar cells with oval nuclei and abundant eosinophilic cytoplasm (Figure 7D) (39). In primary mediastinal synovial sarcomas, a third variant is sometimes recognized, the so called poorly differentiated synovial sarcoma. This subtype consists of highly cellular round or oval cells with hyperchromatic nuclei, rhabdoid cytoplasmic features, high mitotic index and necrosis (Figure 7E) (39).
More than 90% of synovial sarcomas are characterized by a unique t(X; 18)(p11.2; q11.2) translocation resulting in the fusion of the SYT gene on chromosome 18 to either one of three closely related genes, SSX1, SSX2, and SSX4 on chromosome X (41). Hence, molecular testing for this specific translocation is a valuable aid in the workup of these tumors.
By immunohistochemistry, mediastinal synovial sarcomas are characterized by expression of cytokeratins and epithelial membrane antigen in single cells in the monophasic variant and in the glandular component of biphasic tumors (Figure 7F) (39,42,43). More recently, several novel markers have been introduced to identify these tumors. Among these, TLE1 and SS18-SSX are two new antibodies that have been proven useful as a screening tool to identify cases in which molecular genetic testing is likely positive (Figure 7G,7H) (44,45). In this context, it has to be noted that TLE1 is only moderately specific for synovial sarcoma and can be expressed in a range of other neoplasms, including potential mimics (46) while SS18-SSX is highly specific but not completely sensitive. In this scenario, a SSX C-terminus antibody can be added to positively identify synovial sarcomas with non-SS18 fusions or if negative, to exclude the diagnosis altogether (45,47).
Surgery is the primary treatment for mediastinal synovial sarcomas and completeness of resection appears to be the main factor influencing patient survival (48). Patients with these tumors have an unfavorable prognosis with a 5-year overall survival rate of 35.7% compared to 50–80% for synovial sarcomas arising in the soft tissues (48-50). Multiple lines of therapy, including chemotherapy and/or radiotherapy are often used for advanced stage disease, tumor progression and incomplete surgical resection (48,49,51,52).
The monophasic variant of synovial sarcoma is the one most prone to be mistaken for spindle cell thymoma. Both tumors are cellular spindle cell lesions with monomorphic appearance. However, while tumor cells may be rather bland in both entities, those in synovial sarcoma are mitotically active. Furthermore, distinct lobulation, lymphocytic infiltrate and perivascular spaces are features seen in spindle cell thymoma but not in synovial sarcoma. If tumor morphology is inconclusive, immunohistochemistry and/or molecular analyses can be performed and will show strong and diffuse staining with cytokeratin and p63/p40 in spindle cell thymoma while synovial sarcomas will be strongly and diffusely positive for TLE1 and SS18-SSX. Likewise, synovial sarcomas will display SS18::SSX fusion, unlike spindle cell thymoma (Table 2).
Dedifferentiated liposarcoma
Primary mediastinal liposarcomas account for less than 1% of all mediastinal tumors and up to a third of these correspond to the dedifferentiated type (53-59). The latter are histologically characterized by areas of a well differentiated liposarcoma admixed with a sarcoma that lacks obvious adipocytic differentiation by morphologic examination. Dedifferentiated liposarcomas, in particular, can mimic spindle cell thymomas since the dedifferentiated component often presents as an undifferentiated spindle cell neoplasm and the well differentiated liposarcoma component may be inconspicuous. These tumors affect adult patients with a mean age at presentation in the 5th to 6th decade and with equal sex distribution (54,57-60). Cough, dyspnea, dysphagia and chest pain are the most common presenting symptoms, usually due to mass effect on neighboring organs, however, some patients may be entirely asymptomatic despite large tumor size (54,57-60).
Mediastinal liposarcomas are typically large lobulated tumors with a size up to 60 cm (54,58,60). Dedifferentiated liposarcomas will show areas grossly resembling mature fatty tissue representing the well differentiated component juxtaposed with firm solid areas representing the non-lipogenic spindle cell sarcoma (Figure 8A). Areas of hemorrhage and necrosis may be seen in the latter (54,58,60). The microscopic appearance of the non-lipogenic sarcoma can be highly variable and range from less cellular to highly cellular spindle cell proliferations arranged in solid sheets, whorled or hemangiopericytic patterns (Figure 8B). Cytologic atypia, mitotic activity and presence of hemorrhage or necrosis can vary from tumor to tumor (Figure 8C,8D). Likewise, the stroma may range from highly collagenized to myxoid in appearance. The well differentiated component may be inconspicuous or absent and close attention should be paid to the edges of the dedifferentiated elements to identify a rim of background well differentiated liposarcoma composed of mature adipose tissue with variably sized adipocytes, bands of fibrotic stroma and atypical spindle cells that may mimic adipose tissue with reactive changes.
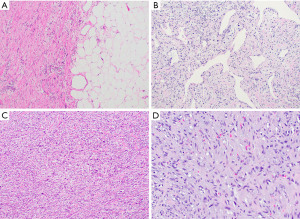
The value of immunohistochemistry for the diagnosis of dedifferentiated liposarcomas remains limited due to an often non-specific immunophenotype. On a molecular level, well differentiated and dedifferentiated liposarcomas are characterized by amplification of the 12q13-15 region, including MDM2 and CDK4 which is detectable by immunohistochemistry and fluorescence in situ hybridization (FISH) (61-64).
As a group, mediastinal liposarcomas are characterized by a high rate of recurrence (approximately 30%) and overall mortality of 30–50% and hence the overall prognosis for these patients remains poor. Complete surgical resection is not only the treatment of choice but is also an important prognostic factor associated with better overall survival (59,65).
Dedifferentiated liposarcoma may closely resemble spindle cell thymoma, particularly if the well differentiated component is not recognized or not sampled sufficiently. If morphological details are inconclusive, immunohistochemical workup is indicated and would support a diagnosis of spindle cell thymoma if diffuse keratin reactivity is seen in combination with expression of p63/p40. On the other hand, if these markers return negative and no other specific immunophenotype is recognized, FISH analysis to identify MDM2 amplification may be indicated and if positive would support a diagnosis of dedifferentiated liposarcoma instead (Table 2).
Miscellaneous other spindle cell neoplasms
There are various other benign and malignant spindle cell neoplasms that can be included in the differential diagnosis of spindle cell thymoma. These are either very rare or more commonly encountered in other mediastinal compartments. Nevertheless, their tumor morphology may show sufficient similarities with that of spindle cell thymoma to be summarized here.
Only few cases of primary angiosarcoma of the mediastinum have been described accounting for less than 1% of mediastinal tumors (66). Many of these tumors are comprised of spindle cells and composed of large, cavernous vascular structures, capillary proliferations, slit-like vascular channels, or solid sheet-like tumor cells (Figure 9A). While low-grade tumors display spindle cells with minimal cytologic atypia and retain the capacity to form vascular structures, high-grade tumors may lose their vasoformative properties. In the latter, necrosis is commonly identified and the mitotic activity is often increased (66,67). Expression of vascular immunohistochemical markers, including factor III-related antigen, CD34, CD31, and erythroblast transformation specific related gene (ERG) is characteristic for these tumors and allows separation from spindle cell thymoma (Figure 9B, Table 2).
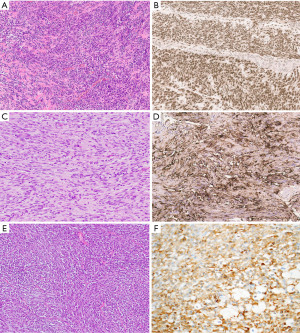
Neurogenic tumors predominantly occur in the posterior mediastinum but can occasionally also arise in the anterior mediastinal compartment (68). Among these, the bland spindle cells of schwannoma are particularly prone to be mistaken for spindle cell thymoma (Figure 9C). Careful search for characteristic Antoni A (high cellularity) and Antoni B (low cellularity) areas and hyalinized vessels will likely point to the correct diagnosis; if needed, immunohistochemical analysis will reveal tumor cells positive for S100, SOX10 and neuron-specific enolase (Figure 9D, Table 2).
Inflammatory myofibroblastic tumors are rare mediastinal lesions, most commonly occurring in young adult patients (69). Due to their composition of bland myofibroblastic spindle cells and diffuse inflammatory cell infiltrate, these tumors may be mistaken for spindle cell thymoma, especially if arising in the anterior mediastinum (Figure 9E). Contrary to spindle cell thymoma, these tumors are characterized immunohistochemically by diffuse expression of vimentin and variable reactivity for smooth muscle actin. Approximately 50% of these tumors will be positive for anaplastic lymphoma kinase (ALK) protein which correlates well with ALK gene rearrangement in these tumors (Figure 9F, Table 2) (70).
The spindle cell variant of mesothelioma (sarcomatoid mesothelioma) may rarely involve the anterior mediastinum. This tumor is composed of fusiform tumor cells arranged in fascicular or storiform patterns and may thus resemble spindle cell thymoma. Close attention should be paid to the cytologic characteristics which will usually demonstrate atypical spindle cells with hyperchromatic nuclei, prominent nucleoli and increased mitotic activity (Figure 10A) (71). The immunophenotype of these tumors is very limited and reactivity is often limited to pancytokeratin and only variable expression of other mesothelial markers (CK5/6, calretinin, WT-1, etc.) (Figure 10B) (72). Contrary to spindle cell thymomas, these tumors are typically negative for p63/p40 (Table 2).
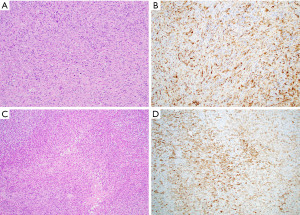
Intimal sarcoma typically arises in the pulmonary arteries of the right ventricular outflow tract of the heart but can involve the anterior mediastinum by direct extension. These tumors can show a wide spectrum of differentiation, ranging from undifferentiated fibroblastic/myofibroblastic tumors to sarcomas with distinct differentiation, such as rhabdomyosarcoma or angiosarcoma among others (Figure 10C) (73). These tumors are often hypercellular and will show varying degrees of cytologic atypia, necrosis and mitotic activity which should serve to distinguish them from spindle cell thymoma. In addition, close attention should be paid to the tumor origin as demonstrated on radiologic imaging, gross and microscopic examination. Immunohistochemically, these tumors will show a variable phenotype depending on the tumor subtype (Figure 10D); however, these neoplasms are generally negative for cytokeratin, CK5/6 and p63/p40 allowing distinction from spindle cell thymoma. Another diagnostic tool includes amplification of MDM2 which is a characteristic alteration in the majority of these tumors (Table 2) (74).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-22-50/coif). AW serves as an unpaid editorial board member of Mediastinum from October 2021 to September 2023. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Weissferdt A. Mesenchymal Tumors of the Mediastinum. In: Weissferdt A. Diagnostic Thoracic Pathology. 1st ed. Cham: Springer; 2020:972.
- Weissferdt A, Moran CA. The histomorphologic spectrum of spindle cell thymoma. Hum Pathol 2014;45:437-45. [Crossref] [PubMed]
- Ströbel P, Badve S, Chan JKC, et al. Type A thymoma. In: World Health Organization. WHO Classification of Tumours Editorial Board. Thoracic Tumours. 5th ed. Lyon: IARC Press; 2021:326-31.
- Nonaka D, Rosai J. Is there a spectrum of cytologic atypia in type a thymomas analogous to that seen in type B thymomas? A pilot study of 13 cases. Am J Surg Pathol 2012;36:889-94. [Crossref] [PubMed]
- Vladislav IT, Gökmen-Polar Y, Kesler KA, et al. The role of histology in predicting recurrence of type A thymomas: a clinicopathologic correlation of 23 cases. Mod Pathol 2013;26:1059-64. [Crossref] [PubMed]
- Moran CA, Kalhor N, Suster S. Invasive spindle cell thymomas (WHO Type A): a clinicopathologic correlation of 41 cases. Am J Clin Pathol 2010;134:793-8. [Crossref] [PubMed]
- Weissferdt A, Hernandez JC, Kalhor N, et al. Spindle cell thymomas: an immunohistochemical study of 30 cases. Appl Immunohistochem Mol Morphol 2011;19:329-35. [Crossref] [PubMed]
- Weissferdt A, Moran CA. Pax8 expression in thymic epithelial neoplasms: an immunohistochemical analysis. Am J Surg Pathol 2011;35:1305-10. [Crossref] [PubMed]
- Radovich M, Pickering CR, Felau I, et al. The Integrated Genomic Landscape of Thymic Epithelial Tumors. Cancer Cell 2018;33:244-58.e10. [Crossref] [PubMed]
- Moran CA, Suster S. Neuroendocrine carcinomas (carcinoid tumor) of the thymus. A clinicopathologic analysis of 80 cases. Am J Clin Pathol 2000;114:100-10. [Crossref] [PubMed]
- Gaur P, Leary C, Yao JC. Thymic neuroendocrine tumors: a SEER database analysis of 160 patients. Ann Surg 2010;251:1117-21. [Crossref] [PubMed]
- Filosso PL, Yao X, Ahmad U, et al. Outcome of primary neuroendocrine tumors of the thymus: a joint analysis of the International Thymic Malignancy Interest Group and the European Society of Thoracic Surgeons databases. J Thorac Cardiovasc Surg 2015;149:103-9.e2. [Crossref] [PubMed]
- Gibril F, Chen YJ, Schrump DS, et al. Prospective study of thymic carcinoids in patients with multiple endocrine neoplasia type 1. J Clin Endocrinol Metab 2003;88:1066-81. [Crossref] [PubMed]
- Filosso PL, Yao X, Ruffini E, et al. Comparison of outcomes between neuroendocrine thymic tumours and other subtypes of thymic carcinomas: a joint analysis of the European Society of Thoracic Surgeons and the International Thymic Malignancy Interest Group. Eur J Cardiothorac Surg 2016;50:766-71. [Crossref] [PubMed]
- Ose N, Maeda H, Inoue M, et al. Results of treatment for thymic neuroendocrine tumours: multicentre clinicopathological study. Interact Cardiovasc Thorac Surg 2018;26:18-24. [Crossref] [PubMed]
- Moran CA, Suster S. Spindle-cell neuroendocrine carcinomas of the thymus (spindle-cell thymic carcinoid): a clinicopathologic and immunohistochemical study of seven cases. Mod Pathol 1999;12:587-91. [PubMed]
- Levine GD, Rosai J. A spindle cell varient of thymic carcinoid tumor. A clinical, histologic, and fine structural study with emphasis on its distinction from spindle cell thymoma. Arch Pathol Lab Med 1976;100:293-300. [PubMed]
- Herbst WM, Kummer W, Hofmann W, et al. Carcinoid tumors of the thymus. An immunohistochemical study. Cancer 1987;60:2465-70. [Crossref] [PubMed]
- Modlin IM, Kidd M, Filosso PL, et al. Molecular strategies in the management of bronchopulmonary and thymic neuroendocrine neoplasms. J Thorac Dis 2017;9:S1458-73. [Crossref] [PubMed]
- Crona J, Björklund P, Welin S, et al. Treatment, prognostic markers and survival in thymic neuroendocrine tumours. a study from a single tertiary referral centre. Lung Cancer 2013;79:289-93. [Crossref] [PubMed]
- Suster S, Moran CA. Spindle cell thymic carcinoma: clinicopathologic and immunohistochemical study of a distinctive variant of primary thymic epithelial neoplasm. Am J Surg Pathol 1999;23:691-700. [Crossref] [PubMed]
- Weissferdt A, Moran CA. Thymic carcinoma, part 1: a clinicopathologic and immunohistochemical study of 65 cases. Am J Clin Pathol 2012;138:103-14. [Crossref] [PubMed]
- Zhang L, Liu X, Li X, et al. Diagnosis and surgical treatment of mediastinal solitary fibrous tumor. Asia Pac J Clin Oncol 2017;13:e473-80. [Crossref] [PubMed]
- Witkin GB, Rosai J. Solitary fibrous tumor of the mediastinum. A report of 14 cases. Am J Surg Pathol 1989;13:547-57. [Crossref] [PubMed]
- Demicco EG, Park MS, Araujo DM, et al. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol 2012;25:1298-306. [Crossref] [PubMed]
- Demicco EG, Wagner MJ, Maki RG, et al. Risk assessment in solitary fibrous tumors: validation and refinement of a risk stratification model. Mod Pathol 2017;30:1433-42. [Crossref] [PubMed]
- van de Rijn M, Lombard CM, Rouse RV. Expression of CD34 by solitary fibrous tumors of the pleura, mediastinum, and lung. Am J Surg Pathol 1994;18:814-20. [Crossref] [PubMed]
- Chmielecki J, Crago AM, Rosenberg M, et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet 2013;45:131-2. [Crossref] [PubMed]
- Doyle LA, Vivero M, Fletcher CD, et al. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol 2014;27:390-5. [Crossref] [PubMed]
- Yoshida A, Tsuta K, Ohno M, et al. STAT6 immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors. Am J Surg Pathol 2014;38:552-9. [Crossref] [PubMed]
- Mohajeri A, Tayebwa J, Collin A, et al. Comprehensive genetic analysis identifies a pathognomonic NAB2/STAT6 fusion gene, nonrandom secondary genomic imbalances, and a characteristic gene expression profile in solitary fibrous tumor. Genes Chromosomes Cancer 2013;52:873-86. [Crossref] [PubMed]
- Robinson DR, Wu YM, Kalyana-Sundaram S, et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet 2013;45:180-5. [Crossref] [PubMed]
- Aydemir B, Celik S, Okay T, et al. Intrathoracic giant solitary fibrous tumor. Am J Case Rep 2013;14:91-3. [Crossref] [PubMed]
- Chu X, Zhang L, Xue Z, et al. Solitary fibrous tumor of the pleura: An analysis of forty patients. J Thorac Dis 2012;4:146-54. [PubMed]
- Xiang Y, Tu S, Zhang F. Rapid metastasis of mediastinal solitary fibrous tumor: Report a case. Medicine (Baltimore) 2017;96:e9307. [Crossref] [PubMed]
- Cardillo G, Carbone L, Carleo F, et al. Solitary fibrous tumors of the pleura: an analysis of 110 patients treated in a single institution. Ann Thorac Surg 2009;88:1632-7. [Crossref] [PubMed]
- Milano MT, Singh DP, Zhang H. Thoracic malignant solitary fibrous tumors: A population-based study of survival. J Thorac Dis 2011;3:99-104. [PubMed]
- Terra SBSP, Aesif SW, Maleszewski JJ, et al. Mediastinal Synovial Sarcoma: Clinicopathologic Analysis of 21 Cases With Molecular Confirmation. Am J Surg Pathol 2018;42:761-6. [Crossref] [PubMed]
- Suster S, Moran CA. Primary synovial sarcomas of the mediastinum: a clinicopathologic, immunohistochemical, and ultrastructural study of 15 cases. Am J Surg Pathol 2005;29:569-78. [Crossref] [PubMed]
- Salah S, Salem A. Primary synovial sarcomas of the mediastinum: a systematic review and pooled analysis of the published literature. ISRN Oncol 2014;2014:412527. [Crossref] [PubMed]
- Przybyl J, Sciot R, Rutkowski P, et al. Recurrent and novel SS18-SSX fusion transcripts in synovial sarcoma: description of three new cases. Tumour Biol 2012;33:2245-53. [Crossref] [PubMed]
- Pelmus M, Guillou L, Hostein I, et al. Monophasic fibrous and poorly differentiated synovial sarcoma: immunohistochemical reassessment of 60 t(X;18)(SYT-SSX)-positive cases. Am J Surg Pathol 2002;26:1434-40. [Crossref] [PubMed]
- Miettinen M, Limon J, Niezabitowski A, et al. Calretinin and other mesothelioma markers in synovial sarcoma: analysis of antigenic similarities and differences with malignant mesothelioma. Am J Surg Pathol 2001;25:610-7. [Crossref] [PubMed]
- Foo WC, Cruise MW, Wick MR, et al. Immunohistochemical staining for TLE1 distinguishes synovial sarcoma from histologic mimics. Am J Clin Pathol 2011;135:839-44. [Crossref] [PubMed]
- Zaborowski M, Vargas AC, Pulvers J, et al. When used together SS18-SSX fusion-specific and SSX C-terminus immunohistochemistry are highly specific and sensitive for the diagnosis of synovial sarcoma and can replace FISH or molecular testing in most cases. Histopathology 2020;77:588-600. [Crossref] [PubMed]
- Kosemehmetoglu K, Vrana JA, Folpe AL. TLE1 expression is not specific for synovial sarcoma: a whole section study of 163 soft tissue and bone neoplasms. Mod Pathol 2009;22:872-8. [Crossref] [PubMed]
- Baranov E, McBride MJ, Bellizzi AM, et al. A Novel SS18-SSX Fusion-specific Antibody for the Diagnosis of Synovial Sarcoma. Am J Surg Pathol 2020;44:922-33. [Crossref] [PubMed]
- Syred K, Weissferdt A. Primary mediastinal synovial sarcomas. Mediastinum 2020;4:13. [Crossref] [PubMed]
- Deshmukh R, Mankin HJ, Singer S. Synovial sarcoma: the importance of size and location for survival. Clin Orthop Relat Res 2004;155-61. [Crossref] [PubMed]
- Ulmer C, Kettelhack C, Tunn PU, et al. Synovial sarcoma of the extremities. Results of surgical and multimodal therapy. Chirurg 2003;74:370-4. [Crossref] [PubMed]
- Eilber FC, Brennan MF, Eilber FR, et al. Chemotherapy is associated with improved survival in adult patients with primary extremity synovial sarcoma. Ann Surg 2007;246:105-13. [Crossref] [PubMed]
- Trassard M, Le Doussal V, Hacène K, et al. Prognostic factors in localized primary synovial sarcoma: a multicenter study of 128 adult patients. J Clin Oncol 2001;19:525-34. [Crossref] [PubMed]
- Macchiarini P, Ostertag H. Uncommon primary mediastinal tumours. Lancet Oncol 2004;5:107-18. [Crossref] [PubMed]
- Hahn HP, Fletcher CD. Primary mediastinal liposarcoma: clinicopathologic analysis of 24 cases. Am J Surg Pathol 2007;31:1868-74. [Crossref] [PubMed]
- Evans HL. Liposarcoma: a study of 55 cases with a reassessment of its classification. Am J Surg Pathol 1979;3:507-23. [Crossref] [PubMed]
- Kindblom LG, Angervall L, Svendsen P. Liposarcoma a clinicopathologic, radiographic and prognostic study. Acta Pathol Microbiol Scand Suppl 1975;1-71. [PubMed]
- Boland JM, Colby TV, Folpe AL. Liposarcomas of the mediastinum and thorax: a clinicopathologic and molecular cytogenetic study of 24 cases, emphasizing unusual and diverse histologic features. Am J Surg Pathol 2012;36:1395-403. [Crossref] [PubMed]
- Ortega P, Suster D, Falconieri G, et al. Liposarcomas of the posterior mediastinum: clinicopathologic study of 18 cases. Mod Pathol 2015;28:721-31. [Crossref] [PubMed]
- Chen M, Yang J, Zhu L, et al. Primary intrathoracic liposarcoma: a clinicopathologic study and prognostic analysis of 23 cases. J Cardiothorac Surg 2014;9:119. [Crossref] [PubMed]
- Klimstra DS, Moran CA, Perino G, et al. Liposarcoma of the anterior mediastinum and thymus. A clinicopathologic study of 28 cases. Am J Surg Pathol 1995;19:782-91. [Crossref] [PubMed]
- Pedeutour F, Suijkerbuijk RF, Forus A, et al. Complex composition and co-amplification of SAS and MDM2 in ring and giant rod marker chromosomes in well-differentiated liposarcoma. Genes Chromosomes Cancer 1994;10:85-94. [Crossref] [PubMed]
- Pedeutour F, Forus A, Coindre JM, et al. Structure of the supernumerary ring and giant rod chromosomes in adipose tissue tumors. Genes Chromosomes Cancer 1999;24:30-41. [Crossref] [PubMed]
- Rosai J, Akerman M, Dal Cin P, et al. Combined morphologic and karyotypic study of 59 atypical lipomatous tumors. Evaluation of their relationship and differential diagnosis with other adipose tissue tumors (a report of the CHAMP Study Group). Am J Surg Pathol 1996;20:1182-9. [Crossref] [PubMed]
- Mandahl N, Höglund M, Mertens F, et al. Cytogenetic aberrations in 188 benign and borderline adipose tissue tumors. Genes Chromosomes Cancer 1994;9:207-15. [Crossref] [PubMed]
- Wychulis AR, Payne WS, Clagett OT, et al. Surgical treatment of mediastinal tumors: a 40 year experience. J Thorac Cardiovasc Surg 1971;62:379-92. [Crossref] [PubMed]
- Weissferdt A, Kalhor N, Suster S, et al. Primary angiosarcomas of the anterior mediastinum: a clinicopathologic and immunohistochemical study of 9 cases. Hum Pathol 2010;41:1711-7. [Crossref] [PubMed]
- Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol 1998;22:683-97. [Crossref] [PubMed]
- Boland JM, Colby TV, Folpe AL. Intrathoracic peripheral nerve sheath tumors-a clinicopathological study of 75 cases. Hum Pathol 2015;46:419-25. [Crossref] [PubMed]
- Gorolay V, Jones B. Inflammatory myofibroblastic tumor of mediastinum with esophageal and bronchial invasion: a case report and literature review. Clin Imaging 2017;43:32-5. [Crossref] [PubMed]
- Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 2007;31:509-20. [Crossref] [PubMed]
- Attanoos RL, Gibbs AR. Pathology of malignant mesothelioma. Histopathology 1997;30:403-18. [Crossref] [PubMed]
- Klebe S, Brownlee NA, Mahar A, et al. Sarcomatoid mesothelioma: a clinical-pathologic correlation of 326 cases. Mod Pathol 2010;23:470-9. [Crossref] [PubMed]
- Huo L, Moran CA, Fuller GN, et al. Pulmonary artery sarcoma: a clinicopathologic and immunohistochemical study of 12 cases. Am J Clin Pathol 2006;125:419-24. [Crossref] [PubMed]
- Bode-Lesniewska B, Zhao J, Speel EJ, et al. Gains of 12q13-14 and overexpression of mdm2 are frequent findings in intimal sarcomas of the pulmonary artery. Virchows Arch 2001;438:57-65. [Crossref] [PubMed]
Cite this article as: Weissferdt A. Spindle cell thymoma and its histological mimickers. Mediastinum 2023;7:25.


