
Management of aero-digestive fistulas: the gastroenterologist’s perspective, a narrative review
Introduction
Esophagorespiratory fistulas (ERFs) are abnormal pathologic communications between the gastrointestinal and respiratory tract. Most congenital ERFs are associated with esophageal atresia (EA) while most acquired ERFs are secondary to pulmonary and esophageal malignancies. Benign etiologies of ERFs include infection, foreign bodies, prolonged intubation and iatrogenic surgical injuries of the esophagus or tracheobronchial tree. Patients typically present with dysphagia and/or respiratory symptoms indicative of aspiration, such as, recurrent cough and mild to severe bronchopulmonary infection. Early diagnosis is paramount as is it associated with improved outcomes. Diagnosis is usually suspected by imaging and confirmed with bronchoscopy or upper gastrointestinal endoscopy. Quality of life is significantly reduced due to poor oral intake and 6-month survival is less than 50% (1). Multiple management options exist including oncologic, endoscopic, and surgical approaches (2,3).
The past few years have witnessed rapid advances in therapeutic endoscopy, and many new endoscopic techniques have emerged and are being successfully used to help manage patients with ERFs. Studies evaluating strategies for the management of ERFs are limited to small retrospective studies and case series making it difficult to establish a standard treatment algorithm.
In this manuscript, we will provide an up-to-date review on the current management options for patients with ERFs with a focus on the rapidly evolving role of interventional endoscopy and novel and emerging endoscopic technology including endoscopic suturing, endoscopic vacuum-assisted closure (EVAC) therapy, and over-the-scope clips (OTSCs). We present this article in accordance with the Narrative Review reporting checklist (available at: https://med.amegroups.com/article/view/10.21037/med-22-48/rc).
Methods
Relevant studies regarding the management of ERFs through August 2022 were identified via a PubMed search using different combinations of the following terms: ‘esophagorespiratory fistula’, ‘tracheoesophageal fistula’, ‘esophageal stenting’, ‘over-the-scope clips’, ‘endoscopic suturing’, ‘tissue sealants’, ‘endoscopic vacuum-assisted closure therapy’ and ‘cardiac septal occlusion devices’. Additional papers were identified by reviewing reference lists of relevant publications. Data was extracted based on the relevance to the topic of the manuscript (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | August 1, 2022 |
| Databases | PubMed |
| Search terms used | ‘esophagorespiratory fistula’, ‘tracheoesophageal fistula’, ‘esophageal stenting’, ‘over-the-scope clips’, ‘endoscopic suturing’, ‘tissue sealants’, ‘endoscopic vacuum-assisted closure therapy’, ‘cardiac septal occlusion devices’ |
| Timeframe | 1983–2022 |
| Inclusion and exclusion criteria | Focus was placed on papers in English related to the endoscopic management of esophagorespiratory fistulas from a gastroenterology standpoint. Papers discussing the management from a pulmonologist’s perspective were excluded |
| Selection process | F.N. conducted the literature search. All the authors subsequently discussed and agreed on the literature selection |
Etiology
ERFs may be congenital or acquired. Congenital tracheoesophageal fistula (TEF) is a common congenital anomaly of the respiratory tract with an estimated incidence of 1 in 4,000 live births (4). TEFs usually occur with EA and are classified into 5 distinct phenotypes according to their anatomical configuration (5). In 4% of the cases, congenital TEF occurs without EA (H-type) and may be diagnosed at an older age (6). The epidemiology and management options of congenital TEFs have been extensively described leading to a significant improvement in outcomes. In contrast, less is known regarding the management of acquired TEFs which typically affects the adult population.
Acquired ERFs are divided into benign or malignant etiologies. Nearly 50% of acquired ERFs are benign and are usually diagnosed in patients with tracheostomies or on mechanical ventilation. Pressure necrosis between the anterior wall of the esophagus and the membranous trachea may lead to the development of ERFs. In mechanically ventilated patients, TEFs are reported in up to 3% of cases with risk factors including poor nutritional status, diabetes, steroids, and active infection (7). Following esophagectomy, postoperative leaks and fistulas can develop in up to 8% of patients with a high 3-month mortality rate of 18.2% (8). Other etiologies include radiation therapy, trauma, caustic ingestion, foreign bodies, non-steroidal anti-inflammatory drugs (NSAIDs), or infection (9,10).
Malignant ERFs originate from complications of esophageal, tracheal, bronchopulmonary, or mediastinal malignancy. They are associated with lower rates of resolution and survival compared to benign fistulas (11). Malignant fistulas can result from tumor invasion and disease progression, or complications from cancer treatment (surgery, radiation therapy, or chemotherapy). Antiangiogenic agents may increase the risk of fistula formation (12,13). Around half of all malignant ERFs involve the trachea and slightly more than a third involve the main bronchi. Aspiration rather than tumor progression is the main cause of death in these patients (14). The incidence of fistulas resulting from esophageal cancer is estimated at 5% to 15% (15), and may be as high as 30% in those with advanced disease (16). Fistula formation is associated with highly progressive less differentiated tumors (17). At the time of fistula detection, 90% of patients with ERFs have metastatic disease and mean survival from the time of diagnosis is less than 3 months (17,18). ERFs develop in less than 1% of lung cancer patients and 14.75% of those with tracheal cancer (19).
ERFs may occur anywhere along the esophagus or respiratory tract. They are more frequently found in the upper and middle third of the esophagus (17,20). Proximal ERFs have lower clinical success rates, and higher rates of recurrent aspiration and adverse events compared to distal ERFs. Overall survival is also shorter with proximal ERFs (20). This may be due to the anatomical proximity of the upper esophagus to the trachea allowing for extensive contamination of the lungs in the event of an aspiration.
Presentation
Patients with ERFs can have multiple clinical manifestations according on the underlying etiology, clinical status, the fistula’s size, rate of formation, and location. In ventilated patients, fistulas may present with persistent air leak despite an inflated cuff, inability to wean from the ventilator, and abdominal distention. In non-intubated patients, coughing immediately after swallowing, fevers, recurrent pneumonias, and respiratory secretions mixed with gastric contents should raise concern for ERF (9). Worsening cough with swallowing (Ono’s sign) is present in 81% of patients with ERFs (21). In malignant ERFs, most patients already have symptoms of malignancy such as dysphagia, dyspnea, pain, and weight loss. An increase in dysphagia or dyspnea is highly suggestive of an ERF in these patients. Other symptoms include hemoptysis, and chest pain (22). The average time from symptoms onset to detection in malignant ERF is 7.3±4.25 months (23).
Clinical management
With a median survival of less than three months from the time of diagnosis, prompt multidisciplinary approach including pulmonology, gastroenterology, thoracic surgery, and oncology is required for the management of ERF. The management of malignant ERFs has evolved from supportive care alone, through an era of surgical options, to minimally invasive approaches.
Surgical management
Surgical options include thoracotomy with direct suture closure, primary repair with tissue flap interposition of both esophageal and airway defects, or esophageal diversion with reconstruction. Single-stage primary repair can be performed in most patients with acquired non-malignant ERFs with superior resolution rates compared to endoscopic options (24). In a systematic review of 165 patients with benign recurrent TEFs, success rate was 93.5% in patients who underwent open surgery compared to 84% with endoscopic therapy. Refistulatization rate was also lower at 21% with surgical treatment and 63% with endoscopic treatment (3). While surgical closure of the fistula and resection of the diseased esophageal and pulmonary tissue provides the best opportunity for recovery in malignant ERFs, this is rarely performed as patients have advanced malignancy and are poor surgical candidates by the time of diagnosis. Therapy of patients with ERF of tumorous origin is mainly palliative. Esophageal bypass surgery is a less invasive option that may provide palliation and improve respiratory symptoms and oral intake in these patients (25).
Chemotherapy and radiation therapy
Although malignant ERF is typically seen late in the course of the disease, esophageal cancer patients may occasionally have ERF on presentation. Chemotherapy and radiation are associated with development and worsening of ERFs due to the concern of fistula enlargement from tumor necrosis. However, treating the underlying malignancy can lead to fistula closure in some patients. In a study of 40 patients with esophageal squamous cell carcinoma and malignant ERF, concurrent chemoradiation combined with adequate enteral nutrition support resulted in fistula closure in 80% of patients with a median time from diagnosis to fistula closure of 5 weeks (26). Similarly, Muto et al. reported fistula closure and resumption of oral diet in 16 out of 24 patients with malignant ERFs treated with chemoradiation (27).
Endoscopic management
Many patients with ERF have poor performance status and are therefore non-ideal candidates for surgical repair. In these cases, non-invasive strategies are employed to achieve fistula closure. Endoscopic closure of an ERF represents an important advancement in the treatment of these patients with improved mortality and morbidity compared to surgical interventions (28). Multiple endoscopic options are currently available including esophageal stenting, argon plasma coagulation (APC), fibrin glue, OTSC placement, polyglycolic acid (PGA) sheets and endoscopic suturing (Table 2). Chronic fistulas persisting more than 6 months is a risk factor for failure of endoscopic therapy and larger orifice size is associated with increased mortality (29). Endoscopic therapy involves either closure, covering, or draining techniques. Treatment options can also be used in combination.
Table 2
| Therapy | Advantages | Disadvantages |
|---|---|---|
| Esophageal stents | (I) Most viable and well-studied intervention; (II) nearly 100% technical success rate; (III) clinical success 56% to 100%; (IV) treats associated esophageal stricture; (V) may allow early oral intake | (I) Oversized stents can stretch the lumen resulting in an enlargement of the fistula; (II) potential airway compression; (III) high complication rate at 25–30%; (IV) prone to migration, often requiring additional intervention for stent fixation; (V) prone to severe chest pain and tissue ingrowth/overgrowth |
| Over-the-scope clips | (I) Relatively easy to use and safe; (II) high rate of full-thickness closure; (III) nearly 100% technical success rate; (IV) promising results in small series as part of a combination therapy | (I) Limited long-term efficacy as monotherapy at ~45%; (II) high rate of recurrence approaching 50%; (III) not appropriate for large ERFs due to limited opening diameter; (IV) challenging removal if treatment fails |
| Endoscopic suturing | (I) Safe and enables approximation of tissue margins to reduce the fistula size; (II) suturing material does not need to be removed | (I) Requires familiarity with endoscopic suturing device; (II) increased difficultly due to tight endoluminal space and tangential suturing in the esophagus; (III) low rates of sustained fistula closure in small case series |
| Cardiac septal occlusion device | (I) Promising preliminary data with 77.7% rate of successful fistula closure; (II) epithelialization of the device may occur leading to fistula closure | (I) Airway complications and fistula enlargement reported; (II) decreases airway cross-sectional area; (III) data limited to a few case reports |
| Endoscopic vacuum-assisted closure therapy | (I) Excellent clinical outcomes in upper GI transmural defects with 85% successful closure; (II) promotes tissue healing through multiple mechanisms; (III) ability of regular endoscopic evaluation of the defect | (I) Lack of data specific to chronic esophagorespiratory fistulas; (II) transnasal tube must remain in situ for at least 3–4 weeks; (III) multiple endoscopic sessions are required for periodic replacement of the sponge system increasing procedural costs |
| Tissue sealants | (I) Efficacy demonstrated in pediatric population (55.7% efficacy in fistula resolution); (II) higher success rates when used in combination with other modalities; (III) easy to apply | (I) Success mainly limited to small (<5 mm) fistulas; (II) limited evidence in adults; (III) can damage the working channel of the endoscope; (IV) can cause air embolization; (V) risk of tracheobronchial accumulation and airway plugging from overflow of excessive volumes of glue |
| Argon plasma coagulation | (I) Technically easy and widely available; (II) good overall treatment success | (I) Mainly used as a combination strategy with other techniques; (II) only applied to small fistula orifice; (III) may cause enlargement of the fistula |
| Polyglycolic acid sheets | (I) Potential additional benefit when used in combination with other strategies; (II) easy to apply and does not need to be retrieved | (I) Data limited on a few case reports; (II) repeat procedures are often needed |
ERFs, esophagorespiratory fistulas; GI, gastrointestinal.
Esophageal stenting
Esophageal stents are commonly used in the management of ERFs particularly in fistulas involving the middle and lower third of the esophagus (30). The rationale of the luminal stent is to isolate the defect and divert luminal contents allowing healing of the fistula. The stent of choice for the management of ERF are self-expanding metal stents (SEMS) with a significant reduction in complication rates compared to previously placed plastic stents (31). SEMS may be fully covered (FCSEMS) or partially covered (PCSEMS). The uncovered metal mesh ends of a PCSEMS allow for tissue ingrowth to increase adherence and minimize risk of migration. However, the stent’s inherent risk of stimulating mucosal hyperplasia may lead to challenging stent removal and stricture formation (32). FCSEMS were utilized to avoid these adverse events but have an increased risk of migration (33). Stent migration can occur in up to a third of patients with esophageal FCSEMS resulting in increased healthcare costs, endoscopic reintervention, and adverse events (34). Various techniques have been developed for fixation of FCSEMS. Notably, endoscopic suturing was reported to significantly decrease rate of stent migration (35). In addition, an OTSC designed for stent fixation (stentfix OTSC, Ovesco Endoscopy AG, Tubingen, Germany) was associated with a 76.5% relative risk reduction of stent migration (36).
In a study comparing FCSEMS with endoscopic suturing and PCSEMS without suturing in 74 patients with benign esophageal conditions, the rates of stent migration or clinical success were comparable. However, adverse events were higher in those who had a PCSEMS (46% vs. 21%, P=0.03). PCSEMS placement was associated with increased risk of tissue ingrowth and overgrowth leading to secondary stricture formation and difficulty removing the stents. Therefore, FCSEMS with endoscopic suturing or OTSC fixation may be preferred to prevent migration (37).
SEMS may be placed with direct endoscopic visualization or under fluoroscopic guidance. After advancement of a guidewire through a forward viewing endoscope, a stent delivery system is advanced over the guidewire or through the scope and deployed in adequate position. The stent should cover both the fistula and parts of normal esophageal tissue proximally and distally. Stent location and fistula closure is then confirmed endoscopically and/or with an esophagram. It is important to ensure drainage of any extra-luminal collections prior to stent deployment to reduce septic complications. To note, esophageal stents do not provide an airtight seal over the ERF and the risk of aspiration is still present. In addition, oversized stents can lead to fistula enlargement by stretching the lumen with their radial force.
Technical success is reported in nearly 100% of the cases, however clinical success is variable ranging from 56% to 100% (11,38-42). Upper esophageal location, Eastern Cooperative Oncology Group (ECOG) performance status of 3 or 4, and fistula development during esophageal cancer treatment are independent predictors for initial clinical failure (11,41). After initial clinical success, recurrence was seen in 35% of patients with malignant ERFs (40). Notably, patients with an initial clinical failure following SEMS placement have a significantly worse overall survival compared to those with initial clinical success (11,40). Complication rate is reported at 25% to 30% (11,41), including chest pain, gastroesophageal reflux, tracheal compression, stent migration, tumor overgrowth, upper gastrointestinal (GI) bleeding, fistula neoformation, and perforation. Nearly two thirds of patients experience chest pain after esophageal SEMS placement, which tends to improve within 1 to 2 weeks (43) (Figure 1).
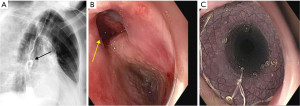
Combined esophageal and airway stenting
Insertion of an esophageal or a tracheobronchial stent is usually sufficient in patients presenting with an ERF. However, combined placement of esophageal and tracheobronchial stents may be entertained for a small subset of patients with ERFs (44). It is estimated that 15% of patients with ERFs could require double stenting (45). This is typically done in situations when an expanding esophageal stent could lead to airway compromise, in patients with preexisting tracheal stenosis, and in large fistulas (>20 mm) (46). However, dual esophageal and airway stenting may lead to worsening of the fistula due to pressure necrosis from the two opposing stents. To minimize the likelihood of injury, a stent with less radial force and small diameter should be selected in these situations. Airway stenting is usually performed first under general anesthesia to avoid potential tracheal compression by the expanding esophageal stent. Parallel esophageal and tracheal stenting is associated with significant improvement in dysphagia and dyspnea (45,47,48). Although the stenting strategies of single vs. combined stents are used based on limited case series, there are no comparative trials assessing the efficacy of both approaches. The main concerns with parallel stenting are airway compromise and major hemorrhage resulting from esophageal tissue necrosis and erosion of tracheal and esophageal walls at sites where stents are in opposition, leading to bleeding from the esophageal venous plexus. Włodarczyk et al. described 31 patients who underwent double stenting with 100% technical success rate and significant improvement in symptoms and quality of life. One patient however had massive fatal bleeding on the third day after stenting (47). Binkert et al. also report on two patients who had fatal hemorrhage 2 and 3 weeks after parallel stenting (49). This technique should be considered carefully as complications can be fatal, if possible, single stenting should be the preferred strategy.
OTSCs
OTSCs were initially developed and successfully used for the management of GI bleeding and perforations (50). OTSCs showed a high rate of full-thickness closure in several studies compared to standard hemoclips (51). Closure of perforations is not hindered by fibrosis. However, successful closure of chronic GI fistulas is considerably more challenging due to edema and inflammation. OTSCs were first used in 2010 for successful closure of an ERF (50). Two OTSC systems are currently used. The Ovesco OTSC (Ovesco Endoscopy AG, Tubingen, Germany) consists of a nitinol clip that is preloaded on an applicator cap attached on the endoscope tip. The clip is deployed by pulling a wire through the endoscope working channel. The clip branches are equipped with teeth that have additional spikes to anchor the clip at the target site while approximating the wound edges. Three types of the clip can be used depending on the teeth configuration. These include the blunt or atraumatic type (a type), the traumatic type with short, pointed teeth (t type), and the traumatic type with long pointed teeth (gc type). A twin grasper or an anchoring device is typically used to approximate the edges of the defect before deploying the clip. The tri-prong anchor device can be particularly helpful if the tissue is indurated or scarred as in ERFs. The Padlock clip (STERIS, Mentor, OH, USA) is another OTSC device with a comparable release mechanism to the Ovesco clip with only one available size.
In a multicenter study, OTSCs were evaluated for the management of GI defects in 188 patients, of which 108 had fistulas and 16 were ERFs. Among those with fistulas, technical success was achieved in 93.4% of the cases and long-term clinical success in 42.9%. Concomitant endoscopic therapy was performed in 43.5% with APC being the most common. All technical failures occurred due to fibrotic or retracted edges of the fistula impending adequate opposition of the defect borders. Clinical success was significantly lower compared to closure of perforation and leaks (52). A review of the published literature including 388 cases with fistulas reported an overall clinical success rate of 52% (53). Silon et al. describe four patients where OTSC was used as part of combination therapy (one with esophageal stent and three with airway stents) all of which achieved clinical success (20). OTSC is considered a relatively safe device with OTSC-related complications estimated at 1.7% and severe complications at 0.59% (53).
Endoscopic suturing
Endoscopic suturing systems represent a minimally invasive endoscopic suturing technique with a wide host of applications in the GI tract. The Overstitch and Overstitch SX (Apollo Endosurgery, Inc., Austin, TX, USA) are currently the main endoscopic suturing platforms commercially available. A double channel upper endoscope is required for the Overstitch while the Overstitch SX can be used with a regular upper endoscope. The suturing system allows placement of sutures in a continuous or interrupted fashion without the need to remove the endoscope for suture reloading. Endoscopic suturing enables the reduction of the size of the leak or fistula by approximation of the opposite tissue margins. Nonetheless, expertise and specific training is required for the use of this device. Suturing may be challenging in ERFs where suturing is tangential with a tight endoluminal space. Before attempting endoscopic suturing of an epithelialized fistula, the chances of successful closure may be improved by de-epithelializing the perimeter of the fistula. Coagulation of the defect perimeter with APC or mechanical abrasion with a brush catheter are the two most common techniques used (54). Pang et al. describe a technique in a patient with TEF consisting of small mucosal resections surrounding the fistula opening to help tissue apposition, and the denuded tissue would reduce the chances of recurrence (55).
There is overall limited literature evaluating the outcomes and efficacy of endoscopic suturing for ERFs. When used, endoscopic suturing is typically part of a multimodal approach. In a multicenter study including 40 patients with GI fistulas, clinical success was reported at 80% (56). Jin et al. report 20 patients with a total of 23 GI fistulas who underwent endoscopic suturing, of which 12 were TEFs. 60% had underwent prior failed attempts at endoscopic closure. Although all patients had concomitant use of APC and achieved technical success, sustained fistula closure was observed in only 5 patients (25%) on surveillance endoscopy at 3 months. In addition, patients with TEF were predisposed to shorter dehiscence-free survival than those with other fistulas (57). Several case reports and case series have described successful ERF closure with endoscopic suturing (58-60). Catalano et al. reported successful closure of 6 different enteric fistulas including 2 ERFs. Notably, these fistulas required 3 to 4 sessions for complete closure (61).
The X-Tack endoscopic HeliX Tacking System (Apollo Endosurgery, Inc., Austin, TX, USA) is a through the scope suture-based device used to close irregular and wide defects in the upper and lower GI tract. In a multicenter study describing the use of this device for GI closure and stent fixation, fistula closure was performed in 11 cases. Technical success with complete fistula closure was achieved in nine cases. Long term follow-up however was not available for most patients (62).
Cardiac septal occlusion devices
Cardiac septal defect occluder (CSO) was developed for percutaneous closure of atrial septal defects and ventricular septal defects. Recently, the device has also been used for endoscopic closure of ERFs. The device consists of two self-expandable polyester coated discs connected by a thin waist and compressed inside a loaded catheter. When deployed, the device closes the luminal contact between the respiratory tract with its waist filling out the fistula itself. The nitinol structure with interwoven polyester liner promotes tissue ingrowth and seals the fistula tract. The disc diameter varices from 9 to 54 mm and the waist size varies from 4 to 38 mm, which can be adjusted to the size of the fistula. The CSO is typically advanced over a guidewire under direct endoscopic visualization with or without fluoroscopic visualization (63). After deployment, each ring expands on either side of the fistula. In a systematic review describing the use of CSO for the management of 22 GI fistulas, including 13 ERFs, technical success was 100% and 77.27% had effective closure after a mean follow-up of 33 weeks. 72.72% had failed endoscopic closure by other modalities. Adverse events occurred in five cases including three migrations, one fistula enlargement, and one migration due to fistula enlargement (64). Multi-patient studies have not been reported to date and further studies are needed to assess the efficacy of this technique. Given that the literature is only limited to case reports, publication bias is a concern as authors tend to publish favorable case reports.
EVAC therapy
EVAC therapy is an innovative endoscopic approach for the management of transmural GI defects (65). It is based on the negative pressure therapy for the management of non-healing wounds and promotes tissue healing through 5 mechanisms including changes in perfusion, microdeformation, macrodeformation, exudate control, and bacterial control (66). The procedure consists of deploying a polyurethane sponge within the fistula attached to the tip of an NG tube. Continuous negative pressure is then applied through the NG tube. The sponge is exchanged every 3 to 5 days. EVAC has shown excellent clinical outcomes in the management of upper GI transmural defects with successful closure in 85% of the cases (67). However, most patients had acute perforations and postsurgical leaks, with only a few reported having bronchoesophageal or TEFs (68-71). Larger studies are needed to further evaluate its role in the management of patients with ERFs.
Tissue sealants
Tissue sealants have successfully been used in the management of anastomotic leaks and fistulas. Most of the evidence for tissue sealants stems from pediatric populations as it has been used in the management of congenital ERFs. Limited data exists in adult patients with acquired TEF. The main classes of sealants are fibrin glue, cyanoacrylate glue, and thrombin (72). Fibrin consists of a solution of fibrinogen and fibrin that when applied, mimics part of the clotting cascade and wound healing. In a study including 52 patients with GI fistulas or anastomotic leaks, including 26 with ERFs, fibrin glue resulted in resolution of the defect in 55.7% of the cases. Between 2 and 81 mL of fibrin was used with a median of 4 sessions. Surgical intervention became necessary in 23.1% (73). Scappaticci et al. describe the successful management of an acquired TEF in a mechanically ventilated patient with fibrin glue (74). Cyanoacrylate glue is a synthetic substance that polymerizes after contact with moisture, causing an inflammatory reaction and tissue necrosis thus inducing tissue healing. Case series have described the successful use of cyanoacrylate glues for the endoscopic treatment of ERFs (75-77). In a systematic review including 203 patients with GI fistulas including 28 ERFs, cumulative success rate was 81%. The majority of ERFs were congenital (78). Tissue sealants used in combination therapy have higher success rates. Richter et al. showed that abrasion techniques combined with tissue sealant (fibrin with added aprotinin) had a high success rate of 93.3% (n=15), compared to 62.5% for abrasion alone (n=8), and 78.6% for sealant alone (n=14) (79). Similarly, fibrin glue combined with electrocautery in patients with recurrent TEF had a higher efficacy compared to electrocautery alone (86% vs. 67%) (80).
Occlusion of the working channel and glue adherence to the tip of the endoscope can lead to instrument damage. Air embolization and death have been reported during fistula treatment with injection of cyanoacrylate and fibrin glues and was possibly related to overinsufflation within the fistula tract (81). When managing ERFs, there is a risk of airway plugging and tracheobronchial accumulation from overflow of excessive volumes of glue (72).
Other endoscopic modalities
Other endoscopic options have been reported in the management of patients with ERFs including PGA sheets, and APC (82-84). These are usually used in association with the previously mentioned endoscopic options.
Several case series have evaluated APC as a treatment option for patients with ERFs (84,85). APC leads to the creation of coagulation-induced inflammation and granulation along the fistula tract. Overall, treatment success was reported in 66% of patients with more than 12 months of follow-up requiring two applications on average (85). Considering the possibility to cause fistula enlargement, this technique should be used with caution.
PGA sheets are suture reinforcement material that are made of a bioabsorbable synthetic polymer and is used in a variety of surgical, pulmonary, and gastroenterology contexts to close fistulas and minimize adverse events at surgical sites (86). A few case reports have described successful resolution of ERFs when PGA sheets were used in combination with fibrin glue, and hemoclips without adverse events (86-88). Based on the limited data, PGA sheets may provide some additional benefit when used for the management of ERFs.
Limitations
Studies evaluating strategies for the management of ERFs are limited to small retrospective studies while head-to-head studies comparing different endoscopic options are lacking. Currently, selecting the endoscopic strategy largely depends on operator preference, location and size of the fistula, viability of the surrounding tissue, and patient’s comorbidities. Future studies comparing the effectiveness of different endoscopic strategies for the management of ERFs could help establish a standardized treatment algorithm and potentially improve patient outcomes.
Conclusions
ERFs are associated with significant morbidity and mortality. A variety of endoscopic therapies are currently available including stent placement, OTSCs, cardiac septal occluder devices, APC, tissue sealants, endoscopic suturing, PGA sheets, and EVAC. With limited data comparing different treatment strategies, it is difficult to establish a standardized therapeutic algorithm. Successful endoscopic management depends on several factors including etiology, lesion chronicity, local tissue viability, comorbidities, and expertise. The management of ERFs remains challenging and may often require multiple treatment modalities. Successful management of ERFs needs a tailored and multidisciplinary approach including surgery, pulmonology, gastroenterology, and oncology.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Bruce Sabath and Roberto F. Casal) for the series “Management of Airway and Vascular Invasion in the Mediastinum” published in Mediastinum. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://med.amegroups.com/article/view/10.21037/med-22-48/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-22-48/coif). The series “Management of Airway and Vascular Invasion in the Mediastinum” was commissioned by the editorial office without any funding or sponsorship. P.S.G. reports that he is a consultant for Boston Scientific and OVESCO. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Qureshi YA, Muntzer Mughal M, Fragkos KC, et al. Acquired adult aerodigestive fistula: classification and management. J Gastrointest Surg 2018;22:1785-94. [Crossref] [PubMed]
- Koike R, Nishimura Y, Nakamatsu K, et al. Concurrent chemoradiotherapy for esophageal cancer with malignant fistula. Int J Radiat Oncol Biol Phys 2008;70:1418-22. [Crossref] [PubMed]
- Aworanti O, Awadalla S. Management of recurrent tracheoesophageal fistulas: a systematic review. Eur J Pediatr Surg 2014;24:365-75. [Crossref] [PubMed]
- Depaepe A, Dolk H, Lechat MF. The epidemiology of tracheo-oesophageal fistula and oesophageal atresia in Europe. EUROCAT Working Group. Arch Dis Child 1993;68:743-8. [Crossref] [PubMed]
- Clark DC. Esophageal atresia and tracheoesophageal fistula. Am Fam Physician 1999;59:910-6, 919-20. [PubMed]
- Sampat K, Losty PD. Diagnostic and management strategies for congenital H-type tracheoesophageal fistula: a systematic review. Pediatr Surg Int 2021;37:539-47. [Crossref] [PubMed]
- Santosham R. Management of Acquired Benign Tracheoesophageal Fistulae. Thorac Surg Clin 2018;28:385-92. [Crossref] [PubMed]
- Rutegård M, Lagergren P, Rouvelas I, et al. Intrathoracic anastomotic leakage and mortality after esophageal cancer resection: a population-based study. Ann Surg Oncol 2012;19:99-103. [Crossref] [PubMed]
- Elser T, Frederick A, Penn E, et al. Benign tracheal esophageal fistula. Operative Techniques in Thoracic and Cardiovascular Surgery 2020;25:27-41. [Crossref]
- Voiosu TA, Gheorghe AV, Bobeica DA, et al. Endoscopic management of recurrent tracheoesophageal fistula induced by chronic use of nonsteroidal anti-inflammatory drugs: A case report and review of the literature. Rom J Intern Med 2018;56:211-5. [Crossref] [PubMed]
- Ribeiro MSI, da Costa Martins B, Simas de Lima M, et al. Self-expandable metal stent for malignant esophagorespiratory fistula: predictive factors associated with clinical failure. Gastrointest Endosc 2018;87:390-6. [Crossref] [PubMed]
- Goodgame B, Veeramachaneni N, Patterson A, et al. Tracheo-esophageal fistula with bevacizumab after mediastinal radiation. J Thorac Oncol 2008;3:1080-1. [Crossref] [PubMed]
- Spigel DR, Hainsworth JD, Yardley DA, et al. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol 2010;28:43-8. [Crossref] [PubMed]
- Burt M, Diehl W, Martini N, et al. Malignant esophagorespiratory fistula: management options and survival. Ann Thorac Surg 1991;52:1222-8; discussion 1228-9. [Crossref] [PubMed]
- Pao TH, Chen YY, Chang WL, et al. Esophageal fistula after definitive concurrent chemotherapy and intensity modulated radiotherapy for esophageal squamous cell carcinoma. PLoS One 2021;16:e0251811. [Crossref] [PubMed]
- Chen B, Deng M, Yang C, et al. High incidence of esophageal fistula on patients with clinical T4b esophageal squamous cell carcinoma who received chemoradiotherapy: A retrospective analysis. Radiother Oncol 2021;158:191-9. [Crossref] [PubMed]
- Balazs A, Kupcsulik PK, Galambos Z. Esophagorespiratory fistulas of tumorous origin. Non-operative management of 264 cases in a 20-year period. Eur J Cardiothorac Surg 2008;34:1103-7. [Crossref] [PubMed]
- Reed MF, Mathisen DJ. Tracheoesophageal fistula. Chest Surg Clin N Am 2003;13:271-89. [Crossref] [PubMed]
- Shamji FM, Inculet R. Management of Malignant Tracheoesophageal Fistula. Thorac Surg Clin 2018;28:393-402. [Crossref] [PubMed]
- Silon B, Siddiqui AA, Taylor LJ, et al. Endoscopic Management of Esophagorespiratory Fistulas: A Multicenter Retrospective Study of Techniques and Outcomes. Dig Dis Sci 2017;62:424-31. [Crossref] [PubMed]
- Gerzić Z, Rakić S, Randjelović T. Acquired benign esophagorespiratory fistula: report of 16 consecutive cases. Ann Thorac Surg 1990;50:724-7. [Crossref] [PubMed]
- Gudovsky LM, Koroleva NS, Biryukov YB, et al. Tracheoesophageal fistulas. Ann Thorac Surg 1993;55:868-75. [Crossref] [PubMed]
- Diddee R, Shaw IH. Acquired tracheo-oesophageal fistula in adults. Continuing Education in Anaesthesia Critical Care & Pain 2006;6:105-8. [Crossref]
- Shen KR, Allen MS, Cassivi SD, et al. Surgical management of acquired nonmalignant tracheoesophageal and bronchoesophageal fistulae. Ann Thorac Surg 2010;90:914-8; discussion 919. [Crossref] [PubMed]
- Nakajima Y, Kawada K, Tokairin Y, et al. Retrospective Analyses of Esophageal Bypass Surgery for Patients with Esophagorespiratory Fistulas Caused by Esophageal Carcinomas. World J Surg 2016;40:1158-64. [Crossref] [PubMed]
- Ma L, Luo GY, Ren YF, et al. Concurrent chemoradiotherapy combined with enteral nutrition support: a radical treatment strategy for esophageal squamous cell carcinoma patients with malignant fistulae. Chin J Cancer 2017;36:8. [Crossref] [PubMed]
- Muto M, Ohtsu A, Miyamoto S, et al. Concurrent chemoradiotherapy for esophageal carcinoma patients with malignant fistulae. Cancer 1999;86:1406-13. [Crossref] [PubMed]
- Meier JD, Sulman CG, Almond PS, et al. Endoscopic management of recurrent congenital tracheoesophageal fistula: a review of techniques and results. Int J Pediatr Otorhinolaryngol 2007;71:691-7. [Crossref] [PubMed]
- Debourdeau A, Gonzalez JM, Dutau H, et al. Endoscopic treatment of nonmalignant tracheoesophageal and bronchoesophageal fistula: results and prognostic factors for its success. Surg Endosc 2019;33:549-56. [Crossref] [PubMed]
- Ross WA, Alkassab F, Lynch PM, et al. Evolving role of self-expanding metal stents in the treatment of malignant dysphagia and fistulas. Gastrointest Endosc 2007;65:70-6. [Crossref] [PubMed]
- Taal BG, Kooyman WM, Boot H. Expandable stents compared to conventional plastic endoprostheses in malignant oesophageal obstruction, especially in cardiac cancer and fistulas: the experience of the Netherlands Cancer Institute. Eur J Gastroenterol Hepatol 1998;10:745-52. [Crossref] [PubMed]
- Gangloff A, Lecleire S, Di Fiore A, et al. Fully versus partially covered self-expandable metal stents in benign esophageal strictures. Dis Esophagus 2015;28:678-83. [Crossref] [PubMed]
- Seven G, Irani S, Ross AS, et al. Partially versus fully covered self-expanding metal stents for benign and malignant esophageal conditions: a single center experience. Surg Endosc 2013;27:2185-92. [Crossref] [PubMed]
- Ko HK, Song HY, Shin JH, et al. Fate of migrated esophageal and gastroduodenal stents: experience in 70 patients. J Vasc Interv Radiol 2007;18:725-32. [Crossref] [PubMed]
- Ngamruengphong S, Sharaiha RZ, Sethi A, et al. Endoscopic suturing for the prevention of stent migration in benign upper gastrointestinal conditions: a comparative multicenter study. Endoscopy 2016;48:802-8. [Crossref] [PubMed]
- Schiemer M, Bettinger D, Mueller J, et al. Reduction of esophageal stent migration rate with a novel over-the-scope fixation device (with video). Gastrointest Endosc 2022;96:1-8. [Crossref] [PubMed]
- Ngamruengphong S, Sharaiha R, Sethi A, et al. Fully-covered metal stents with endoscopic suturing vs. partially-covered metal stents for benign upper gastrointestinal diseases: a comparative study. Endosc Int Open 2018;6:E217-23. [Crossref] [PubMed]
- van Halsema EE, van Hooft JE. Clinical outcomes of self-expandable stent placement for benign esophageal diseases: A pooled analysis of the literature. World J Gastrointest Endosc 2015;7:135-53. [Crossref] [PubMed]
- Sarper A, Oz N, Cihangir C, et al. The efficacy of self-expanding metal stents for palliation of malignant esophageal strictures and fistulas. Eur J Cardiothorac Surg 2003;23:794-8. [Crossref] [PubMed]
- Shin JH, Song HY, Ko GY, et al. Esophagorespiratory fistula: long-term results of palliative treatment with covered expandable metallic stents in 61 patients. Radiology 2004;232:252-9. [Crossref] [PubMed]
- Kim PH, Kim KY, Song HY, et al. Self-Expandable Metal Stent Use to Palliate Malignant Esophagorespiratory Fistulas in 88 Patients. J Vasc Interv Radiol 2018;29:320-7. [Crossref] [PubMed]
- Porumb V, Cozorici A, Andrese E, et al. Palliative treatment of malignant esophagopulmonary fistulas with covered self-expandable metallic stents (SEMSS). A single center experience. Rev Med Chir Soc Med Nat Iasi 2015;119:425-30. [PubMed]
- Reijm AN, Didden P, Bruno MJ, et al. Early pain detection and management after esophageal metal stent placement in incurable cancer patients: A prospective observational cohort study. Endosc Int Open 2016;4:E890-4. [Crossref] [PubMed]
- Herth FJ, Peter S, Baty F, et al. Combined airway and oesophageal stenting in malignant airway-oesophageal fistulas: a prospective study. Eur Respir J 2010;36:1370-4. [Crossref] [PubMed]
- van den Bongard HJ, Boot H, Baas P, et al. The role of parallel stent insertion in patients with esophagorespiratory fistulas. Gastrointest Endosc 2002;55:110-5. [Crossref] [PubMed]
- Nomori H, Horio H, Imazu Y, et al. Double stenting for esophageal and tracheobronchial stenoses. Ann Thorac Surg 2000;70:1803-7. [Crossref] [PubMed]
- Włodarczyk J, Kużdżał J. Double stenting for malignant oesophago-respiratory fistula. Wideochir Inne Tech Maloinwazyjne 2016;11:214-21. [Crossref] [PubMed]
- Schweigert M, Posada-González M, Dubecz A, et al. Recurrent oesophageal cancer complicated by tracheo-oesophageal fistula: improved palliation by means of parallel tracheal and oesophageal stenting. Interact Cardiovasc Thorac Surg 2014;18:190-6. [Crossref] [PubMed]
- Binkert CA, Petersen BD. Two fatal complications after parallel tracheal-esophageal stenting. Cardiovasc Intervent Radiol 2002;25:144-7. [Crossref] [PubMed]
- von Renteln D, Denzer UW, Schachschal G, et al. Endoscopic closure of GI fistulae by using an over-the-scope clip (with videos). Gastrointest Endosc 2010;72:1289-96. [Crossref] [PubMed]
- von Renteln D, Vassiliou MC, Rothstein RI. Randomized controlled trial comparing endoscopic clips and over-the-scope clips for closure of natural orifice transluminal endoscopic surgery gastrotomies. Endoscopy 2009;41:1056-61. [Crossref] [PubMed]
- Haito-Chavez Y, Law JK, Kratt T, et al. International multicenter experience with an over-the-scope clipping device for endoscopic management of GI defects (with video). Gastrointest Endosc 2014;80:610-22. [Crossref] [PubMed]
- Kobara H, Mori H, Nishiyama N, et al. Over-the-scope clip system: A review of 1517 cases over 9 years. J Gastroenterol Hepatol 2019;34:22-30. [Crossref] [PubMed]
- Ge PS, Thompson CC. The Use of the Overstitch to Close Perforations and Fistulas. Gastrointest Endosc Clin N Am 2020;30:147-61. [Crossref] [PubMed]
- Pang M, Mousa O, Werlang M, et al. A hybrid endoscopic technique to close tracheoesophageal fistula. VideoGIE 2018;3:15-6. [Crossref] [PubMed]
- Sharaiha RZ, Kumta NA, DeFilippis EM, et al. A large multicenter experience with endoscopic suturing for management of gastrointestinal defects and stent anchorage in 122 patients: a retrospective review. J Clin Gastroenterol 2016;50:388-92. [Crossref] [PubMed]
- Jin D, Xu M, Huang K, et al. The efficacy and long-term outcomes of endoscopic full-thickness suturing for chronic gastrointestinal fistulas with an Overstitch device: is it a durable closure? Surg Endosc 2022;36:1347-54. [Crossref] [PubMed]
- Bonin EA, Wong Kee Song LM, Gostout ZS, et al. Closure of a persistent esophagopleural fistula assisted by a novel endoscopic suturing system. Endoscopy 2012;44 Suppl 2 UCTN:E8-9.
- Chon SH, Toex U, Plum PS, et al. Successful closure of a gastropulmonary fistula after esophagectomy using the Apollo Overstitch and endoscopic vacuum therapy. Endoscopy 2018;50:E149-50. [Crossref] [PubMed]
- Benson AA, Hakimian D, Jacob H, et al. Closure of a chronic complex tracheoesophageal fistula by using endoscopic suturing. VideoGIE 2021;6:119-21. [Crossref] [PubMed]
- Catalano MF, Sorser SA, Henderson JB, et al. 810 Successful Closure of Enteric Fistulas Using the Apollo Overstitch Suturing System. Gastroenterology 2014;5:S-142-S-143. [Crossref]
- Mahmoud T, Wong Kee Song LM, Stavropoulos SN, et al. Initial multicenter experience using a novel endoscopic tack and suture system for challenging GI defect closure and stent fixation (with video). Gastrointest Endosc 2022;95:373-82. [Crossref] [PubMed]
- Repici A, Presbitero P, Carlino A, et al. First human case of esophagus-tracheal fistula closure by using a cardiac septal occluder (with video). Gastrointest Endosc 2010;71:867-9. [Crossref] [PubMed]
- De Moura DTH, Baptista A, Jirapinyo P, et al. Role of cardiac septal occluders in the treatment of gastrointestinal fistulas: a systematic review. Clin Endosc 2020;53:37-48. [Crossref] [PubMed]
- Loske G, Müller CT. Tips and tricks for endoscopic negative pressure therapy. Chirurg 2019;90:7-14. [Crossref] [PubMed]
- de Moura DTH, de Moura BFBH, Manfredi MA, et al. Role of endoscopic vacuum therapy in the management of gastrointestinal transmural defects. World J Gastrointest Endosc 2019;11:329-44. [Crossref] [PubMed]
- Jung DH, Yun HR, Lee SJ, et al. Endoscopic vacuum therapy in patients with transmural defects of the upper gastrointestinal tract: a systematic review with meta-analysis. J Clin Med 2021;10:2346. [Crossref] [PubMed]
- Palmes D, Kebschull L, Bahde R, et al. Management of Nonmalignant Tracheo- and Bronchoesophageal Fistula after Esophagectomy. Thorac Cardiovasc Surg 2021;69:216-22. [Crossref] [PubMed]
- Still S, Mencio M, Ontiveros E, et al. Primary and rescue endoluminal vacuum therapy in the management of esophageal perforations and leaks. Ann Thorac Cardiovasc Surg 2018;24:173-9. [Crossref] [PubMed]
- Valli PV, Mertens JC, Kröger A, et al. Stent-over-sponge (SOS): a novel technique complementing endosponge therapy for foregut leaks and perforations. Endoscopy 2018;50:148-53. [Crossref] [PubMed]
- Lee HJ, Lee H. Endoscopic vacuum-assisted closure with sponge for esophagotracheal fistula after esophagectomy. Surg Laparosc Endosc Percutan Tech 2015;25:e76-7. [Crossref] [PubMed]
- ASGE Technology Committee. Tissue adhesives: cyanoacrylate glue and fibrin sealant. Gastrointest Endosc 2013;78:209-15. [Crossref] [PubMed]
- Lippert E, Klebl FH, Schweller F, et al. Fibrin glue in the endoscopic treatment of fistulae and anastomotic leakages of the gastrointestinal tract. Int J Colorectal Dis 2011;26:303-11. [Crossref] [PubMed]
- Scappaticci E, Ardissone F, Baldi S, et al. Closure of an iatrogenic tracheo-esophageal fistula with bronchoscopic gluing in a mechanically ventilated adult patient. Ann Thorac Surg 2004;77:328-9. [Crossref] [PubMed]
- Yellapu RK, Gorthi JR, Kiranmayi Y, et al. Endoscopic occlusion of idiopathic benign esophago-bronchial fistula. J Postgrad Med 2010;56:284-6. [Crossref] [PubMed]
- Devière J, Quarre JP, Love J, et al. Self-expandable stent and injection of tissue adhesive for malignant bronchoesophageal fistula. Gastrointest Endosc 1994;40:508-10. [Crossref] [PubMed]
- Barthelemy C, Audigier JC, Fraisse H. A non-tumoral esophago-bronchial fistula managed by isobutyl-2-cyanoacrylate. Endoscopy 1983;15:357-8. [Crossref] [PubMed]
- López J, Rodriguez K, Targarona EM, et al. Systematic review of cyanoacrylate embolization for refractory gastrointestinal fistulae: a promising therapy. Surg Innov 2015;22:88-96. [Crossref] [PubMed]
- Richter GT, Ryckman F, Brown RL, et al. Endoscopic management of recurrent tracheoesophageal fistula. J Pediatr Surg 2008;43:238-45. [Crossref] [PubMed]
- Gregory S, Chun RH, Parakininkas D, et al. Endoscopic esophageal and tracheal cauterization for closure of recurrent tracheoesophageal fistula: a case report and review of the literature. Int J Pediatr Otorhinolaryngol 2017;98:158-61. [Crossref] [PubMed]
- Lange V, Meyer G, Wenk H, et al. Fistuloscopy--an adjuvant technique for sealing gastrointestinal fistulae. Surg Endosc 1990;4:212-6. [Crossref] [PubMed]
- Kawabata H, Sone D, Yamaguchi K, et al. Filling of Polyglycolic Acid Sheets for Closure of Gastrointestinal Fistulas With an Easily Deliverable Technique Using a Guidewire. Gastroenterology Res 2020;13:96-100. [Crossref] [PubMed]
- Nakano Y, Takao T, Morita Y, et al. Endoscopic plombage with polyglycolic acid sheets and fibrin glue for gastrointestinal fistulas. Surg Endosc 2019;33:1795-801. [Crossref] [PubMed]
- Wang H, Luo L, Zhou Y, et al. Argon plasma coagulation combined with covered stent placement for management of tracheobronchial stenoses/occlusions as well as esophagorespiratory fistulas. Zhongguo Fei Ai Za Zhi 2010;13:898-902. [PubMed]
- Yankovic F, Castillo C, Saenz R, et al. Endoscopic argon plasma coagulation in recurrent tracheoesophageal fistula. Clinical series and review of the literature. Gastroenterol Hepatol 2009;32:600-4. [Crossref] [PubMed]
- Tsujii Y, Kato M, Shinzaki S, et al. Polyglycolic acid sheets for repair of refractory esophageal fistula. Endoscopy 2015;47 Suppl 1 UCTN:E39-40.
- Kinoshita S, Nishizawa T, Hisamatsu T, et al. Polyglycolic acid sheet for closure of esophagobronchial fistula in a patient with Behçet's disease. Gastrointest Endosc 2017;85:1094-6. [Crossref] [PubMed]
- Matsuura N, Hanaoka N, Ishihara R, et al. Polyglycolic acid sheets for closure of refractory esophago-pulmonary fistula after esophagectomy. Endoscopy 2016;48 Suppl 1 UCTN:E78-9.
Cite this article as: Nehme F, Ge PS, Coronel E. Management of aero-digestive fistulas: the gastroenterologist’s perspective, a narrative review. Mediastinum 2023;7:34.


