
Imaging evaluation of thymic tumors
Introduction
Imaging plays a critical role in the management of patients with thymoma and thymic carcinoma. Imaging is integral in diagnosis and staging, detection of locally invasive and distant metastases, stratification of patients for therapy, and prognosis evaluation. Imaging also serves to assess treatment response and detect recurrent disease after various treatment modalities. This is particularly important since patients with resected recurrent disease have similar outcomes as patients who do not have recurrent disease (1-3).
Routine imaging modalities
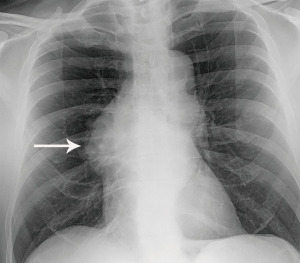
The most performed imaging examination is the routine chest radiograph which can be the first modality suggesting a thymic lesion. Thymic tumors may result in added soft tissue projecting over normal anatomic structures resulting in thickening of the anterior junction line or the “silhouette sign”. Normally air in the lungs delineates the soft tissue structures that abut the lung, such as the heart and mediastinum. Clear delineation of anatomic structures can be limited when a mass is present as soft tissue now abuts normal structure instead of air. This inability to distinguish one structure from another results in obscuration, or the “silhouette sign”. Lateral radiographs help confirm the presence of thymic tumors as they better demonstrate the prevascular space which is readily seen behind the sternum and is normally lucent. A mass in the prevascular space can form a convex contour abnormality when outlined by adjacent lung (Figure 1). Small prevascular lesions may not be radiographically apparent, however. Given the low sensitivity and specificity of radiographs, cross-sectional imaging is essential in the assessment of thymic tumors.
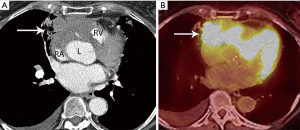
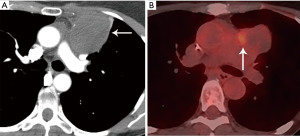
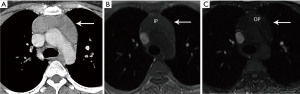
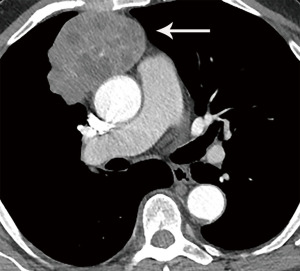
Computed tomography (CT) with contrast is the imaging modality of choice to evaluate thymic tumors due to its high spatial and temporal resolution, ease of access, and convenience. CT can reliably discern location, size/shape, morphology, margins, density, enhancement, and relationship to, or invasion of, adjacent structures (4) (Figure 2). Overall, CT is equal or superior to magnetic resonance imaging (MRI) in the evaluation of mediastinal masses with the caveat that MRI better evaluates thymic cysts or cystic components of tumors (5) (Figure 3).
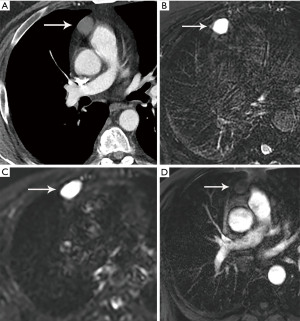
MRI is not routinely utilized in thymic tumor evaluation, although, there are specific scenarios where MRI adds value, such as the differentiation of solid and cystic lesions and the evaluation of cystic or necrotic components of a mass, enhancing septations within a cyst, and the extent of local invasion (Figure 4). Chemical shift imaging can additionally be utilized to detect microscopic or intravoxel fat, which can differentiate thymic neoplasm from hyperplasia (6,7). Finally, MRI can be performed in patients who cannot receive iodinated CT or to avoid radiation exposure.
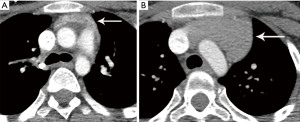
The role of fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) in thymic mass evaluation is incompletely defined. False-positive studies can be seen with FDG uptake in non-neoplastic masses, such as in the setting of infection, thymic hyperplasia, or fibrosing mediastinitis. False-negative studies can be seen in certain histological types of thymic malignancy with lower metabolic activity. Additionally, there is lack of technique standardization which results in quantitative variability between studies (8). Given that other prevascular masses such as malignant germ cell tumor and lymphoma are often FDG avid, the presence of a hypermetabolic prevascular mass cannot distinguish between various tumors. There are studies that report that FDG uptake can help predict tumor invasiveness and prognosis. Other studies report FDG uptake as useful in differentiating low-grade from high-grade thymic malignancies; however, other studies report these observations as controversial due to overlapping imaging findings and FDG uptake between low-grade and high-grade thymic tumors (9). Overlapping findings are less common in more aggressive tumors, such as thymic carcinoma, due to higher overall tumor metabolism, with studies reporting that a maximum standard uptake value (SUVmax) of 6 can serve as a cutoff between thymic carcinoma and lower grade thymic tumors (10) (Figures 2,3). However, this threshold cannot differentiate thymic carcinoma from other malignancies such as lymphoma or non-seminomatous germ cell tumor. Finally, PET/CT clearly has a role to detect occult metastasis in hypermetabolic tumors.
Staging
A variety of thymic tumor staging systems have been utilized over the years. For decades, the predominant staging system, was the Masaoka-Koga staging system as it did correlate with survival (11-13). Masaoka-Koga was based on gross and microscopic pathologic tumor properties. Stage 1 tumors were completely encapsulated. Stage II tumors demonstrating microscopic invasion through the capsule (IIa) or into surrounding fat (IIb). Stage III tumors invade into adjacent structures such as lung, vessels, or pericardium. Finally, stage IV tumors demonstrate pleural or pericardial dissemination (IVa) or lymphatic-hematogenous metastasis (IVb) (1). There were issues with Masaoka-Koga staging, however, such as reliance on an initial small series of 96 patients from one institution and sometimes incomplete or absent capsule limiting stage II evaluation.
Given the need of accurate pre-treatment staging, greater consistency at pathologic examination, and a prognostic determinant on a large patient population from multiple institutions, the International Association for the Study of Lung Cancer (IASLC), the International Thymic Malignancies Interest Group (ITMIG), the European Society of Thoracic Surgeons, the Chinese Alliance for Research on Thymomas, and the Japanese Association of Research on Thymus partnered together to develop a tumor-node-metastasis (TNM) staging system for thymic tumors. While the Masaoka-Koga staging system was derived from retrospective series of 96 patients, the thymic tumor retrospective database included nearly 10,000 patients (14,15). This eighth edition of the TNM classification for malignant tumors has now been adopted by the American Joint Committee on Cancer and the Union for International Cancer Control (16).
In TNM 8th edition, the T descriptor for thymic tumors describes local invasion, and not tumor size, as size was not found to be a prognostic factor. T1 tumors demonstrate invasion into the mediastinal fat (T1a) or mediastinal pleura (T1b), T2 tumors demonstrate invasion into the pericardium, T3 tumors demonstrate invasion into the lung, brachiocephalic vein, superior vena cava (SVC), chest wall, or phrenic nerve, and T4 tumors demonstrate invasion into the aorta, intrapericardial pulmonary artery, myocardium, trachea, or esophagus. The N descriptor distinguishes prevascular or perithymic lymph nodes as N1. Deep intrathoracic or cervical lymph nodes are N2. The M descriptor is divided into M1a disease such as pleural and pericardial nodules and M1b disease such as distant organ metastasis (17-19). While the ultimate assigned stage is often the same with Masaoka-Koga and TNM staging systems, TNM allows for a more detailed breakdown and reporting of the extent of disease (16). A few differences between Masaoka-Koga and TNM will help compare and contrast the two systems. In TNM, capsular and mediastinal pleural invasion are T1 disease, and in the absence of nodal disease would be stage I disease; however, this would be stage II disease in Masaoka-Koga. Pericardial invasion is T2/stage II in TNM, however, in Masaoka-Koga it is stage III. Prevascular or perithymic nodal involvement has been downgraded from Masaoka-Koga stage IVb to TNM stage IVa.
Ried et al. compared the Masaoka-Koga staging system with the TNM staging system. They found that some of the Masaoka-Koga stage IIa and IIb tumors were reclassified to stage I in TNM. Additionally, they reported that the TNM system more accurately characterized Masaoka-Koga stage III disease to determine which patients were better surgical candidates. They concluded that TNM staging was clinically useful and applicable compared with Masaoka-Koga staging (20).
There are, however, limitations of this TNM staging system for thymic tumors. The number of patients evaluated in the initial data who had nonsurgical treatment was small. This could potentially hinder predictive ability with higher stage disease. The nodal map was primarily derived from Japanese practice patterns which are more empirically based with more consideration on feasibility of surgical sampling. Finally, due to the limited data available, differentiation between parenchymal nodules (M1b) and pleural/pericardial nodules (M1a) was an empiric decision lacking statistical validation (16). A revision of this TNM staging is slated to be completed in 2023, revalidating and refining it.
Treatment response assessment
The Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 is currently utilized to assess response to treatment. ITMIG recommends certain modifications and caveats to the use of RECIST version 1.1, however, in thymic tumors due to unique patterns of spread (8). ITMIG recommends pretreatment and posttreatment studies be read by the same radiologist experienced in thoracic imaging to decrease interobserver variability when assessing these often-large irregular tumors with vague borders and local infiltration (21,22). Because thymic tumors tend to spread along the pleura, ITMIG also recommends using the modification of RECIST 1.1 like that used for malignant pleural mesothelioma (MPM) (8,13).
RECIST 1.1, with the modification for MPM, involves measuring up to a maximum of two lesions per organ and five lesions in total, representing all involved organs, as baseline target lesions. Longest diameter measurements are used, with the exception that short axis measurements are obtained of the pleura and lymph nodes. When the pleura is a target organ, short axis (perpendicular to the pleura) measurements are taken at two locations at three separate CT levels separated by at least 1 cm. The sum of these pleural measurements (up to a maximum of 6) becomes the overall pleural measurement, which is added to non-pleural target lesions up to a total of five (8,23).
The National Comprehensive Cancer Network (NCCN) recommends a surveillance regimen of thoracic CT every 6 months for 2 years, then annually for 10 years in thymoma and annually for 5 years in thymic carcinoma. ITMIG, however, recommends surveillance thoracic CT frequency for patients after resection of any thymic tumor as annually for 5 years following resection. From years six to eleven, alternating yearly radiograph and CT is performed, with yearly radiographs thereafter. For resected stage III or IVa thymoma, any stage thymic carcinoma, incomplete resection, or other high-risk tumors, an additional thoracic CT is recommended every six months for the first three years with consideration of CT one to three months after surgery serving as a new baseline after post-surgical changes have resolved (13,24).
CT is the primary modality used for tumor reassessment given that it is the most reproducible (25). When trying to avoid ionizing radiation and in those who cannot be given CT intravenous contrast due to allergy or diminished renal function, MRI can alternatively be used for tumor reassessment. Kerpel et al. evaluated 187 patients after resection for thymic epithelial tumors to assess the accuracy of MRI compared with CT for tumor assessment. MRI was demonstrated to be an adequate alternative to CT for reassessment. One caveat was that due to MRI artifact related to sternotomy wires, alternating CT with MRI was recommended (24).
In patients with microscopically margin negative resection (RO), or in patients who demonstrate complete radiologic response to therapy, local recurrence in the prevascular mediastinum is defined as: tumor in the thymic bed, pericardial, pleural, or pulmonary parenchymal tumor that is immediately adjacent to the thymic bed, lymph nodes immediately adjacent to thymic bed, or in the site of previous noncontiguous metastasis. Regional recurrence is defined as intrathoracic recurrence that is not contiguous with the thymic bed such as: parietal pleural nodules, pericardial nodules, visceral pleural nodules, and lymph nodes not contiguous with the thymic bed. Distal recurrence is defined as: extrathoracic recurrence or intraprenchymal pulmonary nodules that are not contiguous with the thymic bed (13).
Routine imaging characteristics of thymic tumors
Normal anatomic thymus appears as a triangular shaped structure in the prevascular space; however, a variety of normal variants may be seen. Benign and malignant pathologic processes can alter the size or shape of the thymus which can pose a diagnostic dilemma. Generally, benign processes can be easily distinguished based on imaging characteristics. A brief review of benign entities will serve as a comparison to malignant thymic tumors.
Benign
Thymic cysts
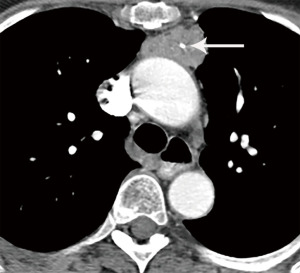
Congenital thymic cysts can occur anywhere along the course of the thymic descent, but most often occur in the prevascular mediastinal space (26). Congenital thymic cysts arise from remnants of the thyropharyngeal duct. Acquired thymic cysts are more common, often multi-locular and complex, associated with neoplasm (such as thymoma, lymphoma, or germ cell tumors), radiation therapy, aplastic anemia, systemic lupus erythematosus, myasthenia gravis, Sjogren syndrome, after tumor resection, an in the setting of acquired immune deficiency syndrome (AIDS) in children (26,27). In general, thymic cysts present as well circumscribed round or oval lesions in the prevascular space with fluid density Hounsfield units (HU) less than 20. They most commonly have no wall thickening, irregularity, or enhancement. If CT findings are indeterminant, MRI can be used for further evaluation. On MRI, simple thymic cysts demonstrate increased T2 signal, variable T1 signal depending on protein content, and no wall nodularity or enhancement (Figure 4).
Thymic hyperplasia
True thymic hyperplasia (or “rebound” hyperplasia) is present when the thymic volume is increased by more than 50%, commonly after infection, surgery, burns, chemotherapy, radiation therapy, or steroid therapy (Figure 5). Alternatively, lymphoid (or follicular) hyperplasia is seen when there is an increase in the number of lymphoid follicles, commonly associated with autoimmune diseases such as myasthenia gravis (Figure 6) or human immunodeficiency virus infection (27-30). There is generally smooth symmetric thymic enlargement in hyperplasia; however, nodular or bulky appearance can be seen which is difficult to distinguish from malignancy. Again, in equivocal cases, MRI can be used in a problem-solving function. In-phase and out-of-phase gradient-echo sequences can identify thymic hyperplasia. Thymic signal drop out during out-of-phase imaging corresponds with microscopic (or intravoxel) fat, which confirms thymic hyperplasia (31) (Figure 6).
Thymolipoma
Thymolipoma is a generally large, benign, slow growing tumor arising from the thymus. Thymolipomas are composed predominantly of adipose tissue with minimal scattered soft tissue and thymic tissue interposed (32,33). The classic appearance is a very large predominantly fat density mass in a cardiophrenic angle, most commonly on the left (Figure 7). Symptoms are overall rare but can be present related to compression or displacement of adjacent structures or in association with Graves’ disease, myasthenia gravis, and hematological disorders (4,30).
Malignant
Thymic epithelial tumors
Thymic epithelial tumors include thymoma, thymic neuroendocrine tumor (carcinoid), and thymic carcinoma. These tumors are predominantly seen in the prevascular mediastinum with a myriad of imaging findings. Thymic epithelial tumors can be homogeneous or heterogeneous, solid or cystic, and have well-circumscribed or irregular borders. There is unfortunately significant imaging overlap between various World Health Organization (WHO) types of thymoma as well as between thymoma and thymic carcinoma. There are, however, clinical and imaging patterns that help in differentiation, but the differentiation usually requires histological proof. Malignant thymic tumors have a median age at presentation in the sixth decade. More benign tumors have a median age at presentation in the fourth to fifth decades (34-36). The more aggressive thymomas and malignant tumors are more likely to have clinical symptoms such as pain or shortness of breath (36). Benign tumors are more likely to have intralesional fat, be midline, and retain normal thymic shape. Malignant tumors are more likely to be larger and locally invasive (37-39). Common imaging findings and techniques utilized to more accurately differentiate various thymic tumors will now be reviewed.
Thymoma
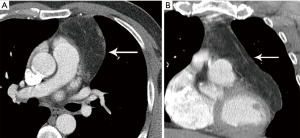
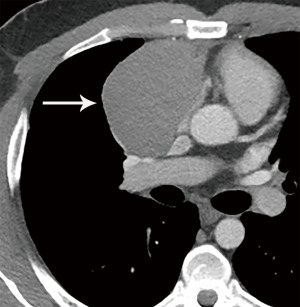
Thymoma typically presents as a smooth or lobular mass involving one lobe of the thymus, although bilateral involvement can occur (39). Most thymomas demonstrate homogeneous enhancement, although, approximately one third can be heterogeneous due to areas of hemorrhage, necrosis, cystic change, or calcification (1) (Figures 8,9). Imaging characteristics can vary according to WHO histological classification, with vascular invasion and pleural/pericardial involvement more common with more aggressive histology (Figure 2). The thymomas with the more aggressive histologies tend to be larger, more lobular or irregular, have cystic or necrotic change, areas of calcification, or evidence of infiltration into surrounding fat (40-42) (Figure 10).
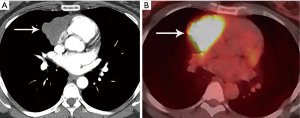
On MRI, thymomas have low to intermediate signal intensity on T1 weighted images and high signal intensity on T2 weighted images. If areas of cystic change or necrosis are present, these have decreased T1 signal intensity and increased T2 signal intensity. Fat suppression imaging can delineate thymomas from mediastinal fat which facilitates more exact measurements and enhancement evaluation. MRI is more limited than CT for calcification detection. MRI strengths include identifying nodules, thickened septae, and/or thickened capsule in cystic thymoma and differentiating cystic thymomas from benign prevascular thymic or pericardial cysts. Because of its superior contrast resolution, MRI also excels in identifying direct cardiac involvement compared to CT (43).
The role of FDG PET/CT in thymoma imaging is limited. Given the presence of FDG uptake in the normal and hyperplastic thymus, especially in younger adults and children, false-positive results can occur. In fact, physiologic uptake has been reported in 28% of patients under 40 years of age and up to 73% in children less than 13 years of age (44). PET/CT has not been shown to differentiate different WHO histological classifications of thymic tumors, although the more aggressive histologies tend towards higher FDG uptake (45,46) (Figures 2,3). Indium111 octreotide nuclear medicine scans have now been replaced by 68Ga-labeled somatostatin analogues because 68Ga-labeled somatostatin analogues, such as 68Ga-DOTATATE, are used for PET/CT, and thus provide better resolution.
Thymic carcinoma and neuroendocrine tumor/carcinoid
Thymic carcinoma and thymic neuroendocrine tumors have similar imaging characteristics which may often overlap with the more aggressive histologies of thymoma, such as B3 thymoma. Thymic carcinomas and neuroendocrine tumors commonly present as large prevascular masses with irregular or poorly marginated borders, areas of necrosis or cystic change, and hemorrhage. Compared with thymomas, there is a greater incidence of local invasion (1) (Figures 2,11). Pleural or pericardial nodules, pleural effusion, and distant metastasis are more commonly seen with thymic carcinoma or thymic neuroendocrine tumor than thymoma (Figure 12). More aggressive thymic epithelial tumors can invade or compress the SVC resulting in SVC syndrome. This is a clinical syndrome marked by swelling of the neck, face, and upper extremities, with associated cough, headache, and shortness of breath. Pleural metastatic disease, which is more common in thymic carcinoma and thymic neuroendocrine carcinoma, generally consists of small enhancing pleural nodules or areas of enhancing pleural thickening. These are generally adequately assessed with thin-slice contrast-enhanced CT, although, contrast-enhanced MRI and PET/CT can be of additional benefit in questionable cases.
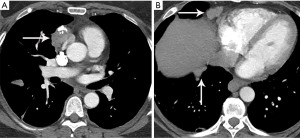
MRI imaging findings of thymic carcinoma and thymic neuroendocrine tumors are similar to thymomas. As already noted, on MRI thymic carcinomas more likely demonstrate irregular contour; greater heterogeneity, hemorrhage, necrosis, and cystic change; greater degree of local vascular and mediastinal invasion; and lymphadenopathy (43,47,48).
As noted, FDG PET/CT does not reliably differentiate between the different histological types of thymoma. Several small studies, however, have suggested that FDG PET/CT can help differentiate thymoma from thymic carcinoma using various cutoffs of SUV max ranging between 4.6 and 6.3 (49,50). Since the more aggressive tumor histologies are FDG avid, FDG PET/CT can be useful in the assessment and follow-up of thymic carcinoma but is not routinely recommended (51). Thymic neuroendocrine tumors can additionally be evaluated with 68Ga-DOTATATE PET/CT which may demonstrate improved sensitivity for lesion detection compared with FDG PET/CT which can help identify tumors that are candidates for peptide receptor radiotherapy (PRRT) with 177Lutetium (51).
Primary thymic salivary gland tumors
Primary salivary gland tumors of the thymus are rare. It is important to differentiate these from more common metastasis by detailed radiologic and clinical evaluation (52). Only a few dozen cases of primary thymic salivary gland tumors, such as adenoid cystic carcinoma and mucoepidermoid carcinoma, have been reported (53-55). Imaging characteristics are like other thymic epithelial neoplasms with final diagnosis depending on histochemical evaluation.
Other prevascular mediastinal masses
Other prevascular lesions can have similar imaging features with thymic epithelial tumors such as lymphoma and germ-cell tumors. Lymphoma may differ though, as Hodgkin and non-Hodgkin lymphomas tend to present as a homogeneous smooth or lobulated soft tissue mass that may surround, but rarely invade adjacent structures.
Germ-cell tumors include a variety of benign and malignant tumors. The most common benign germ-cell tumor is a mature teratoma. Malignant germ-cell tumors include malignant embryonal carcinoma, yolk sac tumor, choriocarcinoma, and mixed germ cell tumor. Malignant germ-cell tumors often present as heterogeneously enhancing masses with areas of cystic change, necrosis, and calcification, which can have overlapping imaging features with thymic epithelial tumors.
Advanced imaging options
CT
To better distinguish various prevascular mediastinal masses, a variety of new CT techniques are being investigated. Functional CT imaging with CT perfusion provides quantitative data on tissue perfusion by acquiring specific graphs for tissue blood flow (BF), blood volume (BV), and permeability surface (PS). These parameters can be used to evaluate tumor angiogenesis, infiltration, and response to therapy (56). Bakan et al. found that CT perfusion values were not significantly different between thymoma and thymic hyperplasia. There were significant differences in BF and BV values between thymomas and malignant prevascular lesions, however, such as thymic carcinoma. This demonstrates a possible role of CT perfusion imaging to aid in differentiation between thymoma and thymic carcinoma (57).
Dual-energy computed tomography (DECT) has also been evaluated to define a role in the differentiation of prevascular masses. Malignant tumors reveal higher iodine concentrations (IC) as compared to benign tumors (58). In a study of 37 patients, Chang et al. found that iodine related Hounsfield units (IHU) and IC could be helpful to differentiate low-risk thymomas, high-risk thymomas, and thymic carcinomas. Lower values were noted in higher grade tumors, presumably due to the presence of necrosis. They concluded that DECT, using iodine concentration measurement derived quantitative analysis, could aid in the ability to differentiate between low-risk thymoma, high-risk thymoma, and thymic carcinoma (59).
MRI
It has been reported that routine MRI cannot reliably differentiate low-risk from high-risk thymic epithelial tumors (48). Non-invasive functional MRI techniques such as diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) values are techniques that allow for quantitative evaluation of thymic epithelial tumors. Razek et al. reported that an ADC cutoff value of 1.22×103 mm2/sec could be used to differentiate low-risk thymoma from high-risk thymoma and thymic carcinoma with an 87% sensitivity, 85% specificity, and 86% accuracy (60). Most commonly hot-spot regions of interest (ROI) are utilized for DWI/ADC measurements. Given concern for errors in sampling, however, several studies have shown that histogram analysis of ADC maps are more accurate and may aid in differentiation between low-risk thymomas, high-risk thymomas, and thymic carcinomas (61,62).
Functional MRI imaging with dynamic contrast enhanced (DCE) MRI has also been studied in relation to prevascular mediastinal tumor evaluation. In a study comparing thymic epithelial tumors, lymphoma, and malignant germ cell tumors, Yabuuchi et al. found that thymic epithelial tumors demonstrated a washout pattern, possibly due to high tumor cellularity and limited stroma, as compared to other tumor types (63,64). In a study evaluating the ability of DCE-MRI to differentiate thymic carcinoma from thymic lymphoma, Shen et al. reported that reflux rate constant from the extracellular extravascular space (EES) to the blood plasma (kep) was lower in thymic carcinoma and that the volume fraction of the EES (ve) was higher in thymic carcinoma as compared to thymic lymphoma (65). While these results are interesting and promising, because of limited study sizes, further research is needed to clarify the role of DCE-MRI to help differentiate between various thymoma types and between thymoma and thymic carcinoma. Finally, early work suggests that quantitative features derived from MRI related to biologic behavior with various radiomics models (advanced mathematical analysis of existing data) may facilitate predictions of pathologic classification and staging of thymic epithelial tumors (66).
Conclusions
Imaging plays an integral role in patients with thymoma and thymic carcinoma. Imaging is used for initial diagnosis and staging, detection of locally invasive disease and distant metastasis, stratification of patients for therapy, and prognostication. Following medical and surgical therapy, imaging helps assess treatment response and to detect recurrent disease. While imaging findings overlap, combining clinical and imaging characteristics of CT, MRI, and PET/CT can help differentiate thymoma and thymic carcinoma, with new CT and MRI techniques currently being evaluated showing potential.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-22-58/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-22-58/coif). EMM reports an honorarium for a lecture from each of the following companies: Boehringer Ingelheim, AstraZeneca, Merck Sharp and Dohme. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Benveniste MF, Rosado-de-Christenson ML, Sabloff BS, et al. Role of imaging in the diagnosis, staging, and treatment of thymoma. Radiographics 2011;31:1847-61; discussion 1861-3. [Crossref] [PubMed]
- Regnard JF, Zinzindohoue F, Magdeleinat P, et al. Results of re-resection for recurrent thymomas. Ann Thorac Surg 1997;64:1593-8. [Crossref] [PubMed]
- Ströbel P, Bauer A, Puppe B, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol 2004;22:1501-9. [Crossref] [PubMed]
- Carter BW, Okumura M, Detterbeck FC, et al. Approaching the patient with an anterior mediastinal mass: a guide for radiologists. J Thorac Oncol 2014;9:S110-8. [Crossref] [PubMed]
- Tomiyama N, Honda O, Tsubamoto M, et al. Anterior mediastinal tumors: diagnostic accuracy of CT and MRI. Eur J Radiol 2009;69:280-8. [Crossref] [PubMed]
- Ackman JB. MR Imaging of Mediastinal Masses. Magn Reson Imaging Clin N Am 2015;23:141-64. [Crossref] [PubMed]
- Ackman JB, Wu CC. MRI of the thymus. AJR Am J Roentgenol 2011;197:W15-20. [Crossref] [PubMed]
- Benveniste MF, Korst RJ, Rajan A, et al. A practical guide from the International Thymic Malignancy Interest Group (ITMIG) regarding the radiographic assessment of treatment response of thymic epithelial tumors using modified RECIST criteria. J Thorac Oncol 2014;9:S119-24. [Crossref] [PubMed]
- Lococo F, Cesario A, Okami J, et al. Role of combined 18F-FDG-PET/CT for predicting the WHO malignancy grade of thymic epithelial tumors: a multicenter analysis. Lung Cancer 2013;82:245-51. [Crossref] [PubMed]
- Benveniste MF, Moran CA, Mawlawi O, et al. FDG PET-CT aids in the preoperative assessment of patients with newly diagnosed thymic epithelial malignancies. J Thorac Oncol 2013;8:502-10. [Crossref] [PubMed]
- Falkson CB, Bezjak A, Darling G, et al. The management of thymoma: a systematic review and practice guideline. J Thorac Oncol 2009;4:911-9. [Crossref] [PubMed]
- Filosso PL, Ruffini E, Lausi PO, et al. Historical perspectives: The evolution of the thymic epithelial tumors staging system. Lung Cancer 2014;83:126-32. [Crossref] [PubMed]
- Huang J, Detterbeck FC, Wang Z, et al. Standard outcome measures for thymic malignancies. J Thorac Oncol 2010;5:2017-23. [Crossref] [PubMed]
- Huang J, Ahmad U, Antonicelli A, et al. Development of the international thymic malignancy interest group international database: an unprecedented resource for the study of a rare group of tumors. J Thorac Oncol 2014;9:1573-8. [Crossref] [PubMed]
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [Crossref] [PubMed]
- Ahmad U. The eighth edition TNM stage classification for thymic tumors: What do I need to know? J Thorac Cardiovasc Surg 2021;161:1524-9.
- Bhora FY, Chen DJ, Detterbeck FC, et al. The ITMIG/IASLC Thymic Epithelial Tumors Staging Project: A Proposed Lymph Node Map for Thymic Epithelial Tumors in the Forthcoming 8th Edition of the TNM Classification of Malignant Tumors. J Thorac Oncol 2014;9:S88-96.
- Kondo K, Van Schil P, Detterbeck FC, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the N and M components for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S81-7. [Crossref] [PubMed]
- Nicholson AG, Detterbeck FC, Marino M, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the T Component for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S73-80. [Crossref] [PubMed]
- Ried M, Eicher MM, Neu R, et al. Evaluation of the new TNM-staging system for thymic malignancies: impact on indication and survival. World J Surg Oncol 2017;15:214. [Crossref] [PubMed]
- Erasmus JJ, Gladish GW, Broemeling L, et al. Interobserver and intraobserver variability in measurement of non-small-cell carcinoma lung lesions: implications for assessment of tumor response. J Clin Oncol 2003;21:2574-82. [Crossref] [PubMed]
- Schwartz LH, Ginsberg MS, DeCorato D, et al. Evaluation of tumor measurements in oncology: use of film-based and electronic techniques. J Clin Oncol 2000;18:2179-84. [Crossref] [PubMed]
- Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol 2004;15:257-60. [Crossref] [PubMed]
- Kerpel A, Beytelman A, Ofek E, et al. Magnetic Resonance Imaging for the Follow-up of Treated Thymic Epithelial Malignancies. J Thorac Imaging 2019;34:345-50. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Quint LE. Imaging of anterior mediastinal masses. Cancer Imaging 2007;7 Spec No A:S56-62.
- Shahrzad M, Le TS, Silva M, et al. Anterior mediastinal masses. AJR Am J Roentgenol 2014;203:W128-38. [Crossref] [PubMed]
- Juanpere S, Cañete N, Ortuño P, et al. A diagnostic approach to the mediastinal masses. Insights Imaging 2013;4:29-52. [Crossref] [PubMed]
- Nasseri F, Eftekhari F. Clinical and radiologic review of the normal and abnormal thymus: pearls and pitfalls. Radiographics 2010;30:413-28. [Crossref] [PubMed]
- Nishino M, Ashiku SK, Kocher ON, et al. The thymus: a comprehensive review. Radiographics 2006;26:335-48. [Crossref] [PubMed]
- Inaoka T, Takahashi K, Mineta M, et al. Thymic hyperplasia and thymus gland tumors: differentiation with chemical shift MR imaging. Radiology 2007;243:869-76. [Crossref] [PubMed]
- Gaerte SC, Meyer CA, Winer-Muram HT, et al. Fat-containing lesions of the chest. Radiographics 2002;22:S61-78. [Crossref] [PubMed]
- Molinari F, Bankier AA, Eisenberg RL. Fat-containing lesions in adult thoracic imaging. AJR Am J Roentgenol 2011;197:W795-813. [Crossref] [PubMed]
- de Jong WK, Blaauwgeers JL, Schaapveld M, et al. Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur J Cancer 2008;44:123-30. [Crossref] [PubMed]
- Engels EA, Pfeiffer RM. Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer 2003;105:546-51. [Crossref] [PubMed]
- McErlean A, Huang J, Zabor EC, et al. Distinguishing benign thymic lesions from early-stage thymic malignancies on computed tomography. J Thorac Oncol 2013;8:967-73. [Crossref] [PubMed]
- Jeong YJ, Lee KS, Kim J, et al. Does CT of thymic epithelial tumors enable us to differentiate histologic subtypes and predict prognosis? AJR Am J Roentgenol 2004;183:283-9. [Crossref] [PubMed]
- Jung KJ, Lee KS, Han J, et al. Malignant thymic epithelial tumors: CT-pathologic correlation. AJR Am J Roentgenol 2001;176:433-9. [Crossref] [PubMed]
- Rosado-de-Christenson ML, Strollo DC, Marom EM. Imaging of thymic epithelial neoplasms. Hematol Oncol Clin North Am 2008;22:409-31. [Crossref] [PubMed]
- Marom EM, Milito MA, Moran CA, et al. Computed tomography findings predicting invasiveness of thymoma. J Thorac Oncol 2011;6:1274-81. [Crossref] [PubMed]
- Priola AM, Priola SM, Di Franco M, et al. Computed tomography and thymoma: distinctive findings in invasive and noninvasive thymoma and predictive features of recurrence. Radiol Med 2010;115:1-21. [Crossref] [PubMed]
- Tomiyama N, Müller NL, Ellis SJ, et al. Invasive and noninvasive thymoma: distinctive CT features. J Comput Assist Tomogr 2001;25:388-93. [Crossref] [PubMed]
- Sadohara J, Fujimoto K, Müller NL, et al. Thymic epithelial tumors: comparison of CT and MR imaging findings of low-risk thymomas, high-risk thymomas, and thymic carcinomas. Eur J Radiol 2006;60:70-9. [Crossref] [PubMed]
- Jerushalmi J, Frenkel A, Bar-Shalom R, et al. Physiologic thymic uptake of 18F-FDG in children and young adults: a PET/CT evaluation of incidence, patterns, and relationship to treatment. J Nucl Med 2009;50:849-53. [Crossref] [PubMed]
- Endo M, Nakagawa K, Ohde Y, et al. Utility of 18FDG-PET for differentiating the grade of malignancy in thymic epithelial tumors. Lung Cancer 2008;61:350-5. [Crossref] [PubMed]
- Sung YM, Lee KS, Kim BT, et al. 18F-FDG PET/CT of thymic epithelial tumors: usefulness for distinguishing and staging tumor subgroups. J Nucl Med 2006;47:1628-34. [PubMed]
- Han J, Lee KS, Yi CA, et al. Thymic epithelial tumors classified according to a newly established WHO scheme: CT and MR findings. Korean J Radiol 2003;4:46-53. [Crossref] [PubMed]
- Inoue A, Tomiyama N, Fujimoto K, et al. MR imaging of thymic epithelial tumors: correlation with World Health Organization classification. Radiat Med 2006;24:171-81. [Crossref] [PubMed]
- Nakagawa K, Takahashi S, Endo M, et al. Can (18)F-FDG PET predict the grade of malignancy in thymic epithelial tumors? An evaluation of only resected tumors. Cancer Manag Res 2017;9:761-8. [Crossref] [PubMed]
- Shibata H, Nomori H, Uno K, et al. 18F-fluorodeoxyglucose and 11C-acetate positron emission tomography are useful modalities for diagnosing the histologic type of thymoma. Cancer 2009;115:2531-8. [Crossref] [PubMed]
- Hephzibah J, Shanthly N, Oommen R. Diagnostic Utility of PET CT in Thymic Tumours with Emphasis on 68Ga-DOTATATE PET CT in Thymic Neuroendocrine Tumour - Experience at a Tertiary Level Hospital in India. J Clin Diagn Res 2014;8:QC01-3. [Crossref] [PubMed]
- Woo WL, Panagiotopoulos N, Gvinianidze L, et al. Primary mucoepidermoid carcinoma of the thymus presenting with myasthenia gravis. J Thorac Dis 2014;6:E223-5. [PubMed]
- Nonaka D, Klimstra D, Rosai J. Thymic mucoepidermoid carcinomas: a clinicopathologic study of 10 cases and review of the literature. Am J Surg Pathol 2004;28:1526-31. [Crossref] [PubMed]
- Wu SG, Li Y, Li B, et al. Unusual combined thymic mucoepidermoid carcinoma and thymoma: a case report and review of literature. Diagn Pathol 2014;9:8. [Crossref] [PubMed]
- Yasuda M, Yasukawa T, Ozaki D, et al. Mucoepidermoid carcinoma of the thymus. Jpn J Thorac Cardiovasc Surg 2006;54:23-6. [Crossref] [PubMed]
- Trojanowska A, Trojanowski P, Bisdas S, et al. Squamous cell cancer of hypopharynx and larynx - evaluation of metastatic nodal disease based on computed tomography perfusion studies. Eur J Radiol 2012;81:1034-9. [Crossref] [PubMed]
- Bakan S, Kandemirli SG, Dikici AS, et al. Evaluation of anterior mediastinal solid tumors by CT perfusion: a preliminary study. Diagn Interv Radiol 2017;23:10-4. [Crossref] [PubMed]
- Lee SH, Hur J, Kim YJ, et al. Additional value of dual-energy CT to differentiate between benign and malignant mediastinal tumors: an initial experience. Eur J Radiol 2013;82:2043-9. [Crossref] [PubMed]
- Chang S, Hur J, Im DJ, et al. Volume-based quantification using dual-energy computed tomography in the differentiation of thymic epithelial tumours: an initial experience. Eur Radiol 2017;27:1992-2001. [Crossref] [PubMed]
- Abdel Razek AA, Khairy M, Nada N. Diffusion-weighted MR imaging in thymic epithelial tumors: correlation with World Health Organization classification and clinical staging. Radiology 2014;273:268-75. [Crossref] [PubMed]
- Guo Y, Kong QC, Zhu YQ, et al. Whole-lesion histogram analysis of the apparent diffusion coefficient: Evaluation of the correlation with subtypes of mucinous breast carcinoma. J Magn Reson Imaging 2018;47:391-400. [Crossref] [PubMed]
- Zhang W, Zhou Y, Xu XQ, et al. A Whole-Tumor Histogram Analysis of Apparent Diffusion Coefficient Maps for Differentiating Thymic Carcinoma from Lymphoma. Korean J Radiol 2018;19:358-65. [Crossref] [PubMed]
- Moran CA, Weissferdt A, Kalhor N, et al. Thymomas I: a clinicopathologic correlation of 250 cases with emphasis on the World Health Organization schema. Am J Clin Pathol 2012;137:444-50. [Crossref] [PubMed]
- Yabuuchi H, Matsuo Y, Abe K, et al. Anterior mediastinal solid tumours in adults: characterisation using dynamic contrast-enhanced MRI, diffusion-weighted MRI, and FDG-PET/CT. Clin Radiol 2015;70:1289-98. [Crossref] [PubMed]
- Shen J, Xue L, Zhong Y, et al. Feasibility of using dynamic contrast-enhanced MRI for differentiating thymic carcinoma from thymic lymphoma based on semi-quantitative and quantitative models. Clin Radiol 2020;75:560.e19-25. [Crossref] [PubMed]
- Xiao G, Rong WC, Hu YC, et al. MRI Radiomics Analysis for Predicting the Pathologic Classification and TNM Staging of Thymic Epithelial Tumors: A Pilot Study. AJR Am J Roentgenol 2020;214:328-40. [Crossref] [PubMed]
Cite this article as: Strange CD, Truong MT, Ahuja J, Strange TA, Patel S, Marom EM. Imaging evaluation of thymic tumors. Mediastinum 2023;7:28.


