
Management of aero-digestive fistulas in adults: the bronchoscopist’s perspective
Introduction
Aero-digestive fistulas (ADFs) refer to pathologic connections between the respiratory and gastrointestinal systems. Fistulas between the esophagus and trachea or a bronchus are the most common and can be congenital or acquired. Acquired fistulas may be due to a benign etiology or caused by a malignancy, most frequently esophageal cancer. Presentation can be varied and non-specific, such as the development of a persistent cough, pneumonia, or malnutrition. ADFs have been associated with increased morbidity and mortality, so it is important to diagnose and intervene upon them early. The optimal management strategy is not known but may involve surgery, esophagoscopy, bronchoscopy, and/or supportive care. This review will provide an overview of ADFs with a focus on malignant causes and bronchoscopic management. In-depth reviews of airway stent types and esophageal interventions are covered in accompanying articles in this special series.
Etiologies
Nearly all ADFs presenting in adulthood are acquired. Most congenital ADFs are associated with esophageal atresia and therefore diagnosed soon after birth (1). However, those without esophageal atresia may have minimal symptoms and present as late as the seventh decade of life (2).
Approximately half of acquired ADFs are caused by benign etiologies while the remaining are associated with malignancies (3). The precise incidence of acquired ADFs is unknown as patients may be asymptomatic or have mild, nonspecific symptoms that did not prompt an evaluation for a fistula. Burt et al. reported that 10% of their patients with malignant ADFs were asymptomatic and diagnosed incidentally or at the time of autopsy (4). Meanwhile, other studies have reported a postmortem diagnosis of a malignant ADF in 1–13% of patients with esophageal cancer (5).
Esophageal cancer accounts for 77–92% of malignant ADFs while lung cancers represent 7–16% of cases (4,6). Moreover, approximately 4–8% of esophageal cancers and 0.3% of lung cancers have been reported to be associated with an ADF (4,6). ADFs due to thyroid cancers and lymphomas are seen to a lesser extent as well as any tumor involving the mediastinum. Malignant disease is typically at an advanced stage at the time of ADF diagnosis with up to 90% of cases already being metastatic when presenting with a fistula (6).
Approximately 75% of acquired ADFs are iatrogenic and develop following esophagectomy or other mediastinal surgery, pressure necrosis from a cuffed endotracheal tube, trauma from endoscopy, airway or esophageal stent erosion, and antineoplastic treatments including chemoradiation and bevacizumab (3,7-10). While antineoplastic treatments have been associated with the development of an ADF, its direct correlation may be overestimated since oncologic treatments can prolong survival and therefore allow more time for a fistula to develop and become symptomatic. Balazs et al. found that 90% of patients who developed an ADF after radiotherapy did so greater than 1 month after treatment (6). Tuberculosis was previously a leading cause of benign fistulas, but incidence has waned over time. Other infections associated with ADFs include histoplasmosis, actinomycosis, and abscesses. Additional benign etiologies include inflammatory diseases like rheumatoid arthritis and inflammatory bowel disease, trauma, caustic ingestion, and broncholiths.
Presentation
The time from symptom onset to diagnosis varies widely between studies and depends on the etiology. The time until diagnosis for benign cases ranges from days to decades. Traumatic cases are often diagnosed 1–2 weeks after the inciting event and endotracheal tube or tracheostomy cuff-related fistulas can be seen after 3–4 weeks of mechanical ventilation (2,11,12). The median time from symptom development to diagnosis of malignant ADFs has been reported between 3 days to 7 months with a range of 0–58 months (4,6,11). The most common symptoms include coughing after swallowing (“Ono’s sign”) in 20–100% of patients, dysphagia in 8–100%, purulent tracheobronchitis in 15–100%, pneumonia in 5–95%, chronic cough in 20–81%, dyspnea in 18–69%, cachexia and malnutrition in 18–60%, aspiration in 23–46%, fever in 21–27%, chest pain in 5–23%, respiratory failure requiring mechanical ventilation in 6–15%, and hemoptysis or hematemesis in 2–3% (4,6,12-17).
Diagnosis
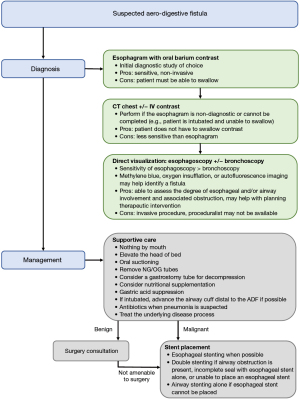
Once an ADF is suspected, the first diagnostic study is typically an esophagram as it is easy to obtain with a sensitivity estimated between 70–97% (17,18). Oral barium sulfate is the contrast of choice over Gastrografin because aspiration of Gastrografin has been associated with fatal pneumonitis and pulmonary edema due to the iodine content as well as the hyperosmolarity of the contrast solution, respectively (19). Computed tomography has a lower reported sensitivity of 50% but may aid in diagnosis when an esophagram is nondiagnostic or cannot be performed due to an inability to swallow contrast (17).
Direct visualization can be performed by esophagoscopy and/or bronchoscopy, which have reported sensitivities of 80% and 46%, respectively (17). These procedures will also help guide treatment strategies, as discussed below. Some fistulas may be difficult to visualize due to their small size or surrounding structural abnormalities. In such cases, methylene blue, autofluorescence imaging, and oxygen insufflation have been used to aid in identifying an ADF (11,20,21).
Fistula location
The location of the ADF will depend on the underlying pathology. Fistulas due to cuff trauma with mechanical ventilation most often involve the trachea. Meanwhile, ADFs due to inflammatory disorders or trauma may occur in any affected area. ADFs following Ivor Lewis esophagectomies have been reported as involving the left mainstem bronchus in 46% of cases and the trachea in 39% (9). With malignant ADFs, the trachea is most frequently involved and comprises 53–80% of cases. Meanwhile, the left mainstem bronchus is involved in 18–25% of cases, the right mainstem bronchus in 2–16%, and the main carina in 7–13% (4,15,22,23). Fistulas occurring at multiple sites have been reported in up to 2% of malignant ADFs (4). This distribution is not unexpected as 64–71% of esophageal tumors are located in the mid-esophagus (6,15).
Classification
A standardized classification of acquired ADFs does not exist although several have been proposed. Wang et al. developed a classification that divides the central airways into 8 zones (14). Zones I–III represent the upper, middle, and lower trachea, respectively; zone IV is the level of the main carina, V the right mainstem bronchus, VI the right bronchus intermedius, VII the proximal left mainstem bronchus, and VIII the distal left mainstem bronchus. In their paper, fistulas were classified as “small” when they measured <10 mm while others have used 5 mm as the threshold to be considered small. Qureshi et al. similarly developed a classification system based on the anatomic location of the ADF (3). In their system, class I fistulas represent those located within 2 cm of the cricoid cartilage, IIa in the proximal and mid-trachea, IIb in the distal trachea, III at the main carina, IV in either mainstem bronchus, and V in more distal airways.
General management
Early intervention is essential as a patient’s quality of life and survival can be significantly impacted by a patent fistula. Mortality is greatest for malignant ADFs with an estimated median survival of 4–8 weeks with medical management alone (4,24,25). However, this may be an underestimation in modern times as these data included patients treated over 30 years ago who were too ill to undergo available interventions of their respective times. The cause of death varies significantly between studies but most commonly include pneumonia with sepsis and respiratory failure (22–82%), malnutrition (1–44%), and hemorrhage (12–33%) (4,26,27). The management of ADFs depends on several factors including the patient’s symptoms and comorbidities, etiology, size and location of the fistula, and local expertise. The primary objective of invasive intervention is to prevent gastrointestinal content spillage into the airways which can cause aspiration pneumonia and sepsis. While the majority of patients will require invasive intervention, some with reversible etiologies like infection or inflammatory bowel disease have demonstrated spontaneous closure by treating the underlying condition (17).
High-quality data regarding different management strategies are limited due to the heterogeneous presentations and the relative rarity of the diagnosis. Nevertheless, the initial aim should be to stabilize and optimize the patient before undergoing any invasive intervention. Respiratory injury from esophageal spillover can be mitigated in several ways. Patients should take in nothing by mouth with oral suctioning as needed and elevate the head of the bed to minimize reflux. Placement of a gastrostomy tube for gastric decompression has also been described (7). Gastric acid suppression using a proton pump inhibitor or H2 blocker should be started. Nasogastric and orogastric tubes should be removed as the added pressure may induce further injury. Mechanically ventilated patients should have the endotracheal tube’s cuff advanced distally to bypass the fistula when possible, which may improve ventilation and reduce soilage of the lower airways. Nutrition can be maintained through a jejunostomy tube or parenterally. Interestingly, one study showed no survival benefit with nutritional supplementation (6). Broad-spectrum antibiotics, including anaerobic coverage, should be initiated if there is suspicion for an infection. Lastly, medical therapies to address the underlying condition should be instituted if they have not already been started.
Surgical intervention
Only a small fraction of ADFs will spontaneously close and invasive intervention is usually needed. Curative surgical management has been recommended when the cause of the fistula is transient or is unlikely to recur (e.g., trauma) as this may provide definitive treatment and reduce the need for future re-intervention (1,28). These patients are often healthier with fewer comorbidities; good outcomes have been reported in 75–93% of cases treated with surgery (28). The mortality associated with surgical repair of benign ADFs is 0–2.8% while morbidity has been reported between 12–54% (11,17,29). In general, surgery should be delayed for patients requiring mechanical ventilation given an increased risk of anastomotic dehiscence and restenosis (7).
A few studies have evaluated surgical intervention for malignant ADFs. Davydov et al. retrospectively assessed 35 patients with ADFs due to esophageal cancer who underwent surgical intervention (30). Post-operative morbidity was 40% and mortality was 14%. The median survival was 13 months with a 2-year survival rate of 21%. Another retrospective study by Lenz et al. included 58 benign and 65 malignant ADFs that were either surgically or endoscopically managed (28). Surgery was the initial intervention in 48% and 11% of patients with benign and malignant ADFs, respectively. With malignant disease, the median survival with surgery was 105 months compared to 2.3 months with non-surgical management. No significant difference was seen between treatment groups with benign ADFs. Of note, survival after surgical intervention of malignant ADFs was higher in this study than reported in other literature, which may be due to baseline patient characteristics as evidenced by a significantly lower Charlson comorbidity index in the surgical group.
Bronchoscopic intervention
In lieu of surgery, several palliative endoscopic and bronchoscopic interventions are available. The most widely used intervention is the placement of esophageal and/or airway stents (Figures 1,2). We will review bronchoscopic management and defer the discussion of endoscopic esophageal management from the gastroenterologist’s perspective to the accompanying article in this series. However, for reasons discussed below, esophageal stenting should be considered first with airway stenting performed if an esophageal stent cannot be placed, there is airway compromise, or there is an inadequate seal with esophageal stenting alone.
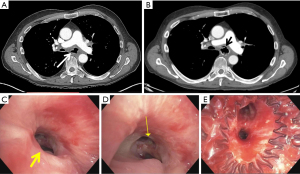
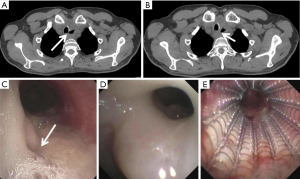
Airway stenting
The type of stent and the decision to place an esophageal stent, airway stent, or both have been evaluated in numerous non-randomized studies. Although esophageal stents were exclusively used when stenting was first introduced as a treatment modality for ADFs, airway stents have become more common over the past two decades. Studies have shown improved fistula occlusion rates with parallel stenting of the esophagus and airway (i.e., “double stenting”) compared to single lumen stenting. Furthermore, there is a possible survival benefit with double stenting or esophageal-only stenting when compared to airway stenting alone (15,23,26,31-33). If double stenting is performed, the proximal end of the esophageal stent should be placed proximal to the airway stent. This will minimize migration and prevent incomplete expansion of the wider end of the esophageal stent, which could result in the stent folding into the esophageal lumen (34). Metal stents are more flexible than silicone stents, which allow them to conform to distorted airways better than silicone stents. Despite this, some have reported less gastric spillover with silicone stents (35). Silicone stents require a rigid bronchoscope for placement which not all patients will tolerate, and advancement of the rigid bronchoscope has the potential to enlarge a fistula.
The American College of Chest Physicians provides a grade 1B recommendation in favor of double stenting malignant tracheoesophageal fistulas (TEFs) with self-expanding metal stents (SEMs) when there is concern for airway compromise (36). Ke et al. reported placing airway stents (70% SEMs and 30% silicone stents) in 61 patients with ADFs, 57% of whom had double stenting performed (35). Two weeks after the procedure, complete occlusion of the fistula was seen in 67% of all patients and in 97% of patients who underwent double stenting. While there was a trend towards improved results with silicone stents compared to SEMs, the difference was not statistically significant. Other studies have reported similar rates of technical success (14,15).
Herth et al. performed a prospective study evaluating stents in the management of malignant ADFs (23). They enrolled 112 patients, 74% of whom had a primary lung cancer while 26% had esophageal cancer. The first stent was placed either in the airway or the esophagus depending on the location of the primary disease. Double stenting was completed if the fistula was inadequately occluded with the first stent, which was assessed with the application of methylene blue during the procedure. Ultimately, 58% of patients received an airway stent alone, 33% received an esophageal stent alone, and 9% received both. Of all patients, 6% required mechanical ventilation for <24 hours following stent placement and there were no incidents of stent migration. ADF recurrence was seen with 26% of airway stents, 16% of esophageal stents, and 10% of double stents, which all occurred >6 weeks after stent placement. The mean survival in those with airway stents alone was significantly lower at 219 days than with esophageal stenting at 262 days and double stenting at 253 days. All patients died as a result of disease progression. Independent risk factors for reduced survival included fistula location in the right mainstem bronchus or those who did not receive additional antineoplastic treatment. Quality of life scores significantly improved immediately following stent placement and persisted for at least 6 weeks. However, it should be noted that this study had a higher proportion of ADFs due to lung cancer than other studies, which likely contributed to a higher percentage of patients who received an airway stent alone.
Several retrospective studies regarding stenting malignant ADFs have also been performed. Freitag et al. compared airway stents to double stenting in 30 patients with malignant TEFs (32). All patients were evaluated for double stenting but 40% only had an airway stent placed either because they were technically unable to place an esophageal stent (e.g., due to a completely obstructed esophagus or prior esophagectomy) or the patient was felt to have such advanced disease that an esophageal stent would not have symptomatically benefited the patient. Following stenting, 97% of patients reported resolution of their dyspnea while 73% had improvement in their dysphagia. Mean survival in the double stenting group was 110 days compared to 24 days in the airway stent group. However, patients who were unable to have an esophageal stent placed may have had poorer baseline prognoses.
In another study by Huang et al., 50 patients with esophageal cancer who had an ADF received either an esophageal stent (42%), an airway stent (26%), or both (32%) depending on the presence of luminal obstruction (15). In those with successful occlusion of the fistula, mean survival was 242 days compared to 80 days in those with stent failure. All patients had significant improvement in their performance status and ultimately died due to progression of their malignancy. Two smaller studies compared airway stenting to supportive care alone and demonstrated increased survival with stenting: 69 vs. 29 days and 120 vs. 55 days (25,33).
When double stenting is planned, airway stenting should be performed prior to esophageal stenting. This will reduce the risk of airway obstruction by the esophageal stent, esophageal spillover, increased airway pressures with esophageal insufflation during esophagoscopy, and air leak with positive pressure ventilation (11). Conversely, an airway stent has the potential to limit full expansion of an esophageal stent (34). When the potential for airway compromise is indeterminate, some have recommended performing an esophagoscopy and inflating a balloon to simulate the effect of a stent while simultaneously performing a bronchoscopy to evaluate for airway compression (37,38).
Overall, studies have consistently demonstrated significant palliation of symptoms following stent placement. While stenting may facilitate receiving additional chemotherapy and/or radiotherapy, there have been mixed reports about the benefit of antineoplastic treatment on survival following stent placement (15,16,23,25,33,39).
Complications of airway stenting
Procedure related mortality is estimated at 0–2.4% (34). Lower respiratory infections have been reported in 38–42% of patients (16,40). In a study by Ost et al., 73 out of 172 patients with airway stents had 106 lower respiratory infections with over half of cases resulting in hospitalization and 23% dying within 2 weeks of their infection (40). Stent migration is seen in 0–20% of cases and is more likely to occur with straight silicone stents than with metal stents. Migration is rarely seen with Y-shaped stents (14,23,32,39,40). Secretion retention requiring intervention is seen in 10–66% of patients, in part due to impaired mucociliary clearance through the stented airways. This is more likely to occur with silicone stents and left mainstem stents (16,39,40). Prophylactic airway clearance therapies should be instituted after stent placement, such as nebulized hypertonic saline, albuterol, and use of a percussive device. Furthermore, 19–31% of patients will develop significant granulation tissue (14,16,40). Other complications include mild chest discomfort in 10–61% of cases, significant intraprocedural hemorrhage in 2–7%, and fistula recurrence in 13–66% (14-16,34,38,39). Overall, new ADF formation due to double stenting is rare although one study reported the development of a new fistula in 3 out of 8 patients (41,42). There have also been cases of double metal stents causing fatal hemorrhage due to erosion into the esophageal venous plexus (14,39,43).
Other bronchoscopic interventions
In addition to stents, the use of various other bronchoscopic interventions has been described in smaller studies and case reports. The AmplatzerTM cardiac septal occluder, a double-disc shaped device made of nitinol and polyester, has been used to successfully occlude benign ADFs (44-46). These devices can be removed once the esophageal side has re-epithelialized but will partially occlude the airway lumen while in place and may not be suitable for patients with existing airway obstruction (47). Cases of device migration into the airway as well as fistula enlargement have been reported (47,48). Chemical cautery, argon plasma coagulation, and lasers have been used to seal small fistulas (35). Fibrin glue has been used for fistulas <5 mm in size but its effect is transient as the glue degrades within 2 weeks (11,49,50). There are case reports of using sutures, a silicone nasal septal button, a cufflink-shaped “DJ” silicone prosthesis, ACell decellularized porcine urinary bladder matrix, dermal filler (calcium hydroxylapatite), glutaraldehyde crosslinked collagen, and cyanoacrylate glue to varying degrees of success (51-56).
Conclusions
ADFs are relatively uncommon but important to recognize given the high associated morbidity and mortality. Interventions to remove or occlude the fistula have been shown to significantly improve a patient’s quality of life and survival. Bronchoscopic interventions are palliative and most often involve placement of an airway stent to occlude the fistula. There is limited data that suggests poorer outcomes with airway stenting alone when compared to esophageal stenting alone or double stenting, but this approach may be necessary in certain circumstances. In the absence of airway involvement, an esophageal stent alone is a reasonable initial management strategy as this is typically better tolerated than an airway stent, and most malignant ADFs will involve some degree of esophageal stenosis. Double stenting should be considered if there is concern for airway compromise after esophageal stent placement, failure of prior esophageal stenting, or fistula formation due to an esophageal stent. See Figure 3 for a proposed management algorithm.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Bruce Sabath and Roberto F. Casal) for the series “Management of Airway and Vascular Invasion in the Mediastinum” published in Mediastinum. The article has undergone external peer review.
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-22-38/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-22-38/coif). The series “Management of Airway and Vascular Invasion in the Mediastinum” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zacharias J, Genc O, Goldstraw P. Congenital tracheoesophageal fistulas presenting in adults: presentation of two cases and a synopsis of the literature. J Thorac Cardiovasc Surg 2004;128:316-8. [Crossref] [PubMed]
- Becker RM, Lespérance R, Despas P, et al. Congenital esophagobronchial fistula in a 62-year-old woman. Chest 1976;69:110-2. [Crossref] [PubMed]
- Qureshi YA, Muntzer Mughal M, Fragkos KC, et al. Acquired Adult Aerodigestive Fistula: Classification and Management. J Gastrointest Surg 2018;22:1785-94. [Crossref] [PubMed]
- Burt M, Diehl W, Martini N, et al. Malignant esophagorespiratory fistula: management options and survival. Ann Thorac Surg 1991;52:1222-8; discussion 1228-9. [Crossref] [PubMed]
- Duranceau A, Jamieson GG. Malignant tracheoesophageal fistula. Ann Thorac Surg 1984;37:346-54. [Crossref] [PubMed]
- Balazs A, Kupcsulik PK, Galambos Z. Esophagorespiratory fistulas of tumorous origin. Non-operative management of 264 cases in a 20-year period. Eur J Cardiothorac Surg 2008;34:1103-7. [Crossref] [PubMed]
- Reed MF, Mathisen DJ. Tracheoesophageal fistula. Chest Surg Clin N Am 2003;13:271-89. [Crossref] [PubMed]
- Spigel DR, Hainsworth JD, Yardley DA, et al. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol 2010;28:43-8. [Crossref] [PubMed]
- Lambertz R, Hölscher AH, Bludau M, et al. Management of Tracheo- or Bronchoesophageal Fistula After Ivor-Lewis Esophagectomy. World J Surg 2016;40:1680-7. [Crossref] [PubMed]
- Zheng B, Zeng T, Yang H, et al. The clinical characteristics, treatments and prognosis of post-esophagectomy airway fistula: a multicenter cohort study. Transl Lung Cancer Res 2022;11:331-41. [Crossref] [PubMed]
- Kim HS, Khemasuwan D, Diaz-Mendoza J, et al. Management of tracheo-oesophageal fistula in adults. Eur Respir Rev 2020;29:200094. [Crossref] [PubMed]
- Diddee R, Shaw IH. Acquired tracheo-oesophageal fistula in adults. Continuing Education in Anaesthesia Critical Care & Pain 2006;6:105-8. [Crossref]
- Colt HG, Meric B, Dumon JF. Double stents for carcinoma of the esophagus invading the tracheo-bronchial tree. Gastrointest Endosc 1992;38:485-9. [Crossref] [PubMed]
- Wang H, Tao M, Zhang N, et al. Airway Covered Metallic Stent Based on Different Fistula Location and Size in Malignant Tracheoesophageal Fistula. Am J Med Sci 2015;350:364-8. [Crossref] [PubMed]
- Huang PM, Lee JM. Are single or dual luminal covered expandable metallic stents suitable for esophageal squamous cell carcinoma with esophago-airway fistula? Surg Endosc 2017;31:1148-55. [Crossref] [PubMed]
- Khan A, Hashim Z, Neyaz Z, et al. Dual Airway and Esophageal Stenting in Advanced Esophageal Cancer With Lesions Near Carina. J Bronchology Interv Pulmonol 2020;27:286-93. [Crossref] [PubMed]
- Mammana M, Pangoni A, Lorenzoni G, et al. Adult Benign, Non-Iatrogenic Bronchoesophageal Fistulae: Systematic Review and Descriptive Analysis of Individual Patient Data. World J Surg 2021;45:3449-57. [Crossref] [PubMed]
- Couraud L, Ballester MJ, Delaisement C. Acquired tracheoesophageal fistula and its management. Semin Thorac Cardiovasc Surg 1996;8:392-9. [PubMed]
- Trulzsch DV, Penmetsa A, Karim A, et al. Gastrografin-induced aspiration pneumonia: a lethal complication of computed tomography. South Med J 1992;85:1255-6. [Crossref] [PubMed]
- Morikawa K, Izawa N, Kida H, et al. Detection of a pinhole-sized bronchoesophageal fistula under bronchoscopic autofluorescence imaging. Thorac Cancer 2021;12:2043-5. [Crossref] [PubMed]
- Levine H, Schonfeld T, Handelsman S, et al. Low flow intermittent bronchoscopic oxygen insufflation to identify occult tracheo-esophageal fistulas. Respir Med 2021;186:106544. [Crossref] [PubMed]
- Choi TK, Siu KF, Lam KH, et al. Bronchoscopy and carcinoma of the esophagus I. Findings of bronchoscopy in carcinoma of the esophagus. Am J Surg 1984;147:757-9. [Crossref] [PubMed]
- Herth FJ, Peter S, Baty F, et al. Combined airway and oesophageal stenting in malignant airway-oesophageal fistulas: a prospective study. Eur Respir J 2010;36:1370-4. [Crossref] [PubMed]
- Fitzgerald RH Jr, Bartles DM, Parker EF. Tracheoesophageal fistulas secondary to carcinoma of the esophagus. J Thorac Cardiovasc Surg 1981;82:194-7. [Crossref] [PubMed]
- Chung FT, Lin HC, Chou CL, et al. Airway ultraflex stenting in esophageal cancer with esophagorespiratory fistula. Am J Med Sci 2012;344:105-9. [Crossref] [PubMed]
- Hu Y, Zhao YF, Chen LQ, et al. Comparative study of different treatments for malignant tracheoesophageal/bronchoesophageal fistulae. Dis Esophagus 2009;22:526-31. [Crossref] [PubMed]
- Rodriguez AN, Diaz-Jimenez JP. Malignant respiratory-digestive fistulas. Curr Opin Pulm Med 2010;16:329-33. [Crossref] [PubMed]
- Lenz CJ, Bick BL, Katzka D, et al. Esophagorespiratory Fistulas: Survival and Outcomes of Treatment. J Clin Gastroenterol 2018;52:131-6. [Crossref] [PubMed]
- Shen KR, Allen MS, Cassivi SD, et al. Surgical management of acquired nonmalignant tracheoesophageal and bronchoesophageal fistulae. Ann Thorac Surg 2010;90:914-8; discussion 919. [Crossref] [PubMed]
- Davydov M, Stilidi I, Bokhyan V, et al. Surgical treatment of esophageal carcinoma complicated by fistulas. Eur J Cardiothorac Surg 2001;20:405-8. [Crossref] [PubMed]
- Nam DH, Shin JH, Song HY, et al. Malignant esophageal-tracheobronchial strictures: parallel placement of covered retrievable expandable nitinol stents. Acta Radiol 2006;47:3-9. [Crossref] [PubMed]
- Freitag L, Tekolf E, Steveling H, et al. Management of malignant esophagotracheal fistulas with airway stenting and double stenting. Chest 1996;110:1155-60. [Crossref] [PubMed]
- Chen YH, Li SH, Chiu YC, et al. Comparative study of esophageal stent and feeding gastrostomy/jejunostomy for tracheoesophageal fistula caused by esophageal squamous cell carcinoma. PLoS One 2012;7:e42766. [Crossref] [PubMed]
- Hürtgen M, Herber SCA. Treatment of malignant tracheoesophageal fistula. Thorac Surg Clin 2014;24:117-27. [Crossref] [PubMed]
- Ke M, Wu X, Zeng J. The treatment strategy for tracheoesophageal fistula. J Thorac Dis 2015;7:S389-97. [PubMed]
- Simoff MJ, Lally B, Slade MG, et al. Symptom management in patients with lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e455S-97S.
- Zori AG, Jantz MA, Forsmark CE, et al. Simultaneous dual scope endotherapy of esophago-airway fistulas and obstructions. Dis Esophagus 2014;27:428-34. [Crossref] [PubMed]
- Youness HA, Harris K, Awab A, et al. Bronchoscopic advances in the management of aerodigestive fistulas. J Thorac Dis 2018;10:5636-47. [Crossref] [PubMed]
- Włodarczyk J, Kużdżał J. Double stenting for malignant oesophago-respiratory fistula. Wideochir Inne Tech Maloinwazyjne 2016;11:214-21. [Crossref] [PubMed]
- Ost DE, Shah AM, Lei X, et al. Respiratory infections increase the risk of granulation tissue formation following airway stenting in patients with malignant airway obstruction. Chest 2012;141:1473-81. [Crossref] [PubMed]
- Nomori H, Horio H, Imazu Y, et al. Double stenting for esophageal and tracheobronchial stenoses. Ann Thorac Surg 2000;70:1803-7. [Crossref] [PubMed]
- Bi Y, Ren J, Chen H, et al. Combined airway and esophageal stents implantation for malignant tracheobronchial and esophageal disease: A STROBE-compliant article. Medicine (Baltimore) 2019;98:e14169. [Crossref] [PubMed]
- Binkert CA, Petersen BD. Two fatal complications after parallel tracheal-esophageal stenting. Cardiovasc Intervent Radiol 2002;25:144-7. [Crossref] [PubMed]
- Rabenstein T, Boosfeld C, Henrich R, et al. First use of ventricular septal defect occlusion device for endoscopic closure of an esophagorespiratory fistula using bronchoscopy and esophagoscopy. Chest 2006;130:906-9. [Crossref] [PubMed]
- Coppola F, Boccuzzi G, Rossi G, et al. Cardiac septal umbrella for closure of a tracheoesophageal fistula. Endoscopy 2010;42:E318-9. [Crossref] [PubMed]
- Traina M, Amata M, De Monte L, et al. Chronic tracheoesophageal fistula successfully treated using Amplatzer septal occluder. Endoscopy 2018;50:1236-7. [Crossref] [PubMed]
- Jiang P, Liu J, Yu D, et al. Closure of Nonmalignant Tracheoesophageal Fistula Using an Atrial Septal Defect Occluder: Case Report and Review of the Literature. Cardiovasc Intervent Radiol 2015;38:1635-9. [Crossref] [PubMed]
- Miller PE, Arias S, Lee H, et al. Complications associated with the use of the amplatzer device for the management of tracheoesophageal fistula. Ann Am Thorac Soc 2014;11:1507-9. [Crossref] [PubMed]
- Wiseman NE. Endoscopic closure of recurrent tracheoesophageal fistula using Tisseel. J Pediatr Surg 1995;30:1236-7. [Crossref] [PubMed]
- Scappaticci E, Ardissone F, Baldi S, et al. Closure of an iatrogenic tracheo-esophageal fistula with bronchoscopic gluing in a mechanically ventilated adult patient. Ann Thorac Surg 2004;77:328-9. [Crossref] [PubMed]
- Mozer AB, Michel E, Gillespie C, et al. Bronchoendoscopic Repair of Tracheoesophageal Fistula. Am J Respir Crit Care Med 2019;200:774-5. [Crossref] [PubMed]
- Schmitz S, Van Damme JP, Hamoir M. A simple technique for closure of persistent tracheoesophageal fistula after total laryngectomy. Otolaryngol Head Neck Surg 2009;140:601-3. [Crossref] [PubMed]
- Diaz-Jimenez JP. New Cufflink-Shaped Silicon Prosthesis for the Palliation of Malignant Tracheobronchial-Esophageal Fistula. J Bronchol 2005;12:207-9. [Crossref]
- Mahajan AK, Newkirk M, Rosner C, et al. Successful endobronchial treatment of a non-healing tracheoesophageal fistula from a previous histoplasmosis capsulatum infection using decellularized porcine urinary bladder matrix†. J Surg Case Rep 2018;2018:rjy187. [Crossref] [PubMed]
- Kasbekar AV, Sherman IW. Closure of minor tracheoesophageal fistulae with calcium hydroxlapatite. Auris Nasus Larynx 2013;40:491-2. [Crossref] [PubMed]
- Sharma M, Somani P, Sunkara T, et al. Trans-Tracheal Cyanoacrylate Glue Injection for the Management of Malignant Tracheoesophageal Fistula. Am J Gastroenterol 2018;113:800. [Crossref] [PubMed]
Cite this article as: Chang CH, Lin J. Management of aero-digestive fistulas in adults: the bronchoscopist’s perspective. Mediastinum 2023;7:33.


