
Immunosuppressive treatment for myasthenia gravis crises improve the taste disorder in patients with thymoma: two case reports
Highlight box
Key findings
• We reported two cases of taste disorder in patients with thymoma, who did not regain taste after extended thymectomy but showed improvement after immunosuppressive treatment for myasthenia gravis (MG) crises.
What is known and what is new?
• A few reports showed the taste disorder in patients with thymoma and MG has recovered not after surgery for thymoma but after immunosuppressive treatment for MG. However, worsening taste disorder after recovery is rarely reported.
What is the implication, and what should change now?
• An autoimmunological factor might be associated with this phenomenon as taste disorders were recovered with the immunosuppressive drug. More case reports are required for further confirmation of this rare condition.
Introduction
Taste disorders disturb the quality of life. Moreover, they are associated with many neurological diseases, including myasthenia gravis (MG). In previous case reports, taste disorders were reported in MG patients with (1,2) or without thymoma (3).
We reported two cases of taste disorder in patients with thymoma, who did not regain taste after extended thymectomy but showed improvement after immunosuppressive treatment for MG crises. We present this article in accordance with the CARE reporting checklist (available at https://med.amegroups.com/article/view/10.21037/med-23-8/rc).
Case presentations
Case 1
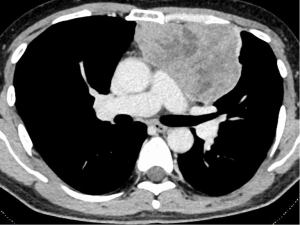
A male in his 50s visited a local hospital for fever and taste disorder, especially for the loss of sweet taste. He expressed his experiences as “I feel chocolate tastes like sand.” Chest computed tomography (CT) revealed an anterior mediastinal mass measuring 10 cm in maximal diameter (Figure 1). He was referred to Nagoya University Hospital for further examination and treatment. A CT-guided percutaneous transthoracic needle biopsy showed type B3 thymoma. Moreover, the 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)-CT scan showed high accumulation in mediastinal mass [the maximum standardized uptake-value (SUVmax): 5.89]. The clinical stage was Masaoka stage III because of the suspected invasion of the left upper lobe (LUL). No subjective symptoms were observed despite the elevated serum concentration of anti-acetylcholine receptor antibody (AchR-Ab) (6.1 nmol/L, normal range: <0.3 nmol/L). The level of plasma albumin before surgery was within the normal limit. He was on no medications before the surgery. In 2014 (before the COVID-19 pandemic), an extended thymectomy combined resection with LUL, left phrenic nerve, and left brachiocephalic vein was performed via median sternotomy and left thoracotomy (at fourth intercostal space). Pathological examination confirmed Masaoka stage II type B3 thymoma. The postoperative course was uneventful, and the patient was discharged from our hospital on postoperative day 8. There was no improvement in taste disorder after surgical resection of the thymoma. Forty-four days after surgery, the patient visited the emergency unit for dysphagia and shortness of breath. The patient was diagnosed with MG crisis and underwent intensive treatment with mechanical ventilation, steroid pulse therapy [methylprednisolone (mPSL), 1,000 mg/day/body for 3 days], and intravenous immunoglobulin (IVIG) therapy (20 g/day/body for 3 days). The patient regained taste when he started oral food intake after the treatment for a MG crisis (about 3 months after surgery). Despite the recovery of taste after steroid pulse therapy and IVIG therapy, taste disorder gradually worsened about 1 year and 9 months after surgery, resulting in an almost complete loss of sweet taste 2 years after surgery. The patient is alive without recurrence of thymoma, 7 years after surgery, without the recovery of taste.
Case 2
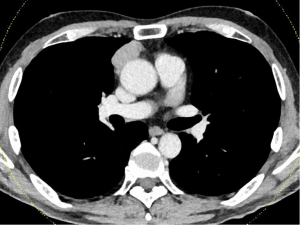
A male in his 60s with an anterior mediastinal mass was referred to Nagoya University Hospital for further examination. He experienced taste disorders including complete loss of sweet, salty, and bitter taste with diminished sour taste just after he was referred to our hospital. He complained as, “I feel that Japanese rice wine (sake) tastes like water.” Chest CT examination revealed an anterior mediastinal mass measuring 5.5 cm in maximal diameter (Figure 2). FDG PET-CT scan showed moderate accumulation in mediastinal mass (SUVmax: 4.45). He showed no symptoms suggesting MG nor elevation in AchR-Ab level (<0.2 nmol/L). Preoperative plasma albumin, serum iron, zinc, vitamin B2, and vitamin B12 levels were within normal limits. He was on no medications before the surgery. He underwent an extended thymectomy via median sternotomy in 2015 (before the COVID-19 pandemic). Pathological examination revealed Masaoka stage II type B1 thymoma. The patient was discharged from the hospital on postoperative day 6. Despite no improvement in taste disorder after surgical thymoma resection, ptosis and double vision appeared 5 years and 2 months after surgery. Moreover, the serum concentration of AchR-Ab was slightly elevated (1.1 nmol/L, normal range, <0.3 nmol/L), and he was diagnosed with MG three months after ptosis and double vision developed; treatment with pyridostigmine (180 mg/body/day) and prednisolone (15 mg/body/day) was initiated. Thereafter, symptoms worsened, resulting in a MG crisis 5 years and 6 months after surgery. There was no change in taste disorder before the MG crisis. After steroid pulse therapy (mPSL, 1,000 mg/day/body), the symptoms have disappeared. Taste disorders gradually recovered within a week. After 6 years and 10 months of surgery, the patient is still alive without MG symptoms (only pyridostigmine, 180 mg/body/day), taste disorder, and thymoma recurrence. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Taste disorder in patients with MG is a relatively rare phenomenon. Even though taste disorders are not life-threatening, they can disturb patients’ quality of life. Therefore, it is essential to treat taste disorders. Chabwine et al. reported a 42-year-old male with MG and thymoma accompanied by loss of sweet taste before treatment (1). They reported that loss of sweet taste in the patient has recovered after immunosuppressive and surgical treatment. Similarly, Kimura et al. reported a 47-year-old female with MG and thymoma accompanied by taste dysgeusia, including reduced perception of sweet taste and increased perception of salty taste, which was not recovered after surgery for thymoma but improved after immunosuppressive treatment for MG (2). A multi-institutional study by Kabasawa et al. showed that taste disorders associated with MG were observed in 9 out of 371 (2.4%) patients with MG (4). All patients had thymoma, which tended to be advanced stage. Loss of sweet taste was more commonly reported than salty, bitter, and sour taste loss. It is unclear which factor, such as the presence of thymoma or MG, is strongly associated with taste disorders in these patients. However, we speculated that the presence of MG or another autoimmune factor would be associated with this phenomenon, as taste disorder did not recover after surgery for thymoma but improved by immunosuppressive treatment in our two cases which were consistent with the reports of a female patient with pure red cell aplasia and thymoma accompanied with taste loss before treatment by Kosaka et al. (3). The reasons for worsening of taste disorders in case 1 and not in case 2 are still unclear. Longer follow-up period is warranted to check if an autoimmune factor, such as autoantibody, is associated with this phenomenon.
The mechanisms of taste disorders in patients with thymoma and MG remains unclear. One possible mechanism is the production of antibodies by the thymus against specific receptors (5). Another possibility is that the thymus produces nicotinic receptors at the neuromuscular junction and muscarinic receptors on taste receptor cells (6). Therefore, more cases should be investigated to confirm the underlying mechanism.
Conclusions
We presented sporadic cases of taste disorder in patients with thymoma, which showed improvement after steroid pulse therapy for MG crises. Although underlying mechanisms remain unclear, an autoimmunological factor might be associated with this phenomenon as taste disorders were recovered with the immunosuppressive drug. More case reports are required for further confirmation of this rare condition.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://med.amegroups.com/article/view/10.21037/med-23-8/rc
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-23-8/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-23-8/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. Approval for this retrospective study was obtained from the institutional review board of Nagoya University Hospital (approval number: 2021-0236).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chabwine JN, Tschirren MV, Zekeridou A, et al. Sweet taste loss in myasthenia gravis: more than a coincidence? Orphanet J Rare Dis 2014;9:50. [Crossref] [PubMed]
- Kimura M, Nakagawa H, Niwa JI, et al. A 47-Year-Old Japanese Woman with Symptoms of Increased Salty and Reduced Sweet Taste Perception Preceding a Diagnosis of Thymoma-Associated Myasthenia Gravis. Am J Case Rep 2022;23:e936000. [Crossref] [PubMed]
- Kosaka T, Nakahashi H, Nakazawa S, et al. Taste disorder in a patient with invasive thymoma without myasthenia gravis: a rare case report. Mediastinum 2022;6:9. [Crossref] [PubMed]
- Kabasawa C, Shimizu Y, Suzuki S, et al. Taste disorders in myasthenia gravis: a multicenter cooperative study. Eur J Neurol 2013;20:205-7. [Crossref] [PubMed]
- Nakazato Y, Ito Y, Naito S, et al. Dysgeusia limited to sweet taste in myasthenia gravis. Intern Med 2008;47:877-78. [Crossref] [PubMed]
- Kiba A, Takeyama H, Unai Y, et al. A case report of myasthenia gravis with taste disorder limited to sweetness. Sumitomo Byouin Igaku Zassi 2013;40:42-6.
Cite this article as: Fukumoto K, Ohara Y, Okado S, Watanabe H, Noritake O, Nakanishi K, Kadomatsu Y, Ueno H, Kato T, Nakamura S, Chen-Yoshikawa TF. Immunosuppressive treatment for myasthenia gravis crises improve the taste disorder in patients with thymoma: two case reports. Mediastinum 2023;7:40.


