Giant middle mediastinal lesions: when tumor size correlates with mesenchymal origin—a retrospective single-center analysis
Highlight box
Key findings
• All giant middle mediastinal tumors—defined as ≥73 mm on axial CT imaging—were of mesenchymal origin in our cohort.
What is known and what is new?
• The prevalence for middle mediastinal tumors is low and best management is unclear.
• The definition “giant” could capture exclusively mesenchymal tumors.
What is the implication, and what should change now?
• Our definition for giant middle mediastinal tumor had a diagnostic impact and could select all patients with tumors of mesenchymal origin.
Introduction
Many different classifications are available to divide the mediastinum (1,2). One of the most accepted classifications is a computed tomography (CT)-based classification, proposed by the International Thymic Malignancy Interest Group (ITMIG) in 2014 (3). This classification divides the mediastinum into three compartments: a prevascular or anterior compartment, a visceral or middle compartment and a paravertebral or posterior compartment (3). Middle or visceral compartment is limited superiorly by the thoracic inlet, inferiorly by the diaphragm, anteriorly by the anterior aspect of the pericardium and posteriorly by an imaginary vertical line located one centimeter posterior to the anterior margin of the thoracic vertebral bodies (3). The middle mediastinal compartment contains the trachea/carina, oesophagus, mediastinal lymph nodes, heart, aorta, superior vena cava, intrapericardial pulmonary arteries and thoracic duct (3). Most common abnormalities located in the middle mediastinum are lymphadenopathy, foregut duplication cysts (bronchogenic, oesophageal) and pericardial cysts. Tracheal, oesophageal and cardiac tumors as well as vascular lesions (aortic aneurysm) may also be encountered in the middle compartment (2).
There is no common definition for giant lesions in general or more specifically for giant lesions of the middle mediastinal compartment. Based on the common familiar definition, the adjective “giant” implies an extremely large size, when compared to similar persons or things. Interestingly, giant lesions may describe specific tumor entities within an organ or a compartment. Focusing on pulmonary malignant lesions, primary pulmonary sarcomas are commonly giant in size compared to primary lung cancer (4).
Here, we defined the term “giant” in the context of a large middle mediastinal lesion and explore the usefulness of such a definition. Our surgical experience in treating patients with giant lesions of the middle mediastinum was described. We present this article in accordance with the STROBE reporting checklist (available at https://med.amegroups.com/article/view/10.21037/med-22-49/rc).
Methods
We performed a retrospective cross-sectional study, including all consecutive patients who underwent surgery (surgical biopsy or resection) for mediastinal lesions from January 2016 to August 2021 in our center. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of University Duisburg-Essen (No. 21-10261-BO) and informed patient consent was waived due to the retrospective analysis.
CT imaging was reviewed by three authors (Kaman H), including two senior surgeons (Collaud S, Stork T) to select patients with isolated lesions located to the middle mediastinal compartment as defined by the three-compartment ITMIG classification (3). Authors were blinded to clinical data as the CT were evaluated. Patients with generalized lymph nodes enlargement (e.g., sarcoidosis) or isolated lymph node enlargement in the context of lung cancer were excluded. In case of large lesions overarching other compartments, lesions were categorized in the compartment of origin, based on the “center method” and “structure displacement tool” described elsewhere (3).
Statistical analysis
Patients’ clinical data were retrieved from electronic charts in our institutional database. It included, operation reports, imaging reports, and pathology report. In patients with suspected sarcoma, histology examination was routinely performed by a dedicated sarcoma pathologist. Lesion size was measured at its largest diameter on axial CT imaging at diagnosis. Distribution curve of lesion size showed a normal distribution. Giant middle mediastinal lesions were therefore defined as middle mediastinal lesions having a size equal or above the 90th percentile of our middle mediastinal lesion cohort. The 90th percentile was selected as it routinely defines upper outliers in different contexts (5-7). Patients with giant middle mediastinal lesions were further described.
Results
One hundred and fifty-seven patients with mediastinal lesions were operated on between January 2016 and August 2021. Thirty-six patients (23%) had lesions located in the middle mediastinal compartment.
Most common diagnoses were mediastinal cysts (n=10, 28%), metastatic lesions (n=6, 17%), lymphomas (n=5, 14%), and sarcomas (n=3, 8%). Other lesions included hemangiomas (n=2, 6%), ectopic thyroids (n=2, 6%), Langerhans cell sarcoma (n=1, 3%), leiomyoma (n=1, 3%), lymphangioma (n=1, 3%), parasitic infection (n=1, 3%), ganglioneuroma (n=1, 3%), thymoma recurrence (n=1, 3%), vagus nerve schwannoma (n=1, 3%) and seminoma (n=1, 3%). Median lesion size was 53 mm (range, 10 to 120 mm). Ninetieth percentile lesion size was 73 mm. As per our definition, four patients had giant middle mediastinal lesions. Patients’ treatment and outcome are described below. For patients with sarcoma, treatment decision was made on a case-by-case basis during our multidisciplinary tumorboard specialized for sarcomas.
Patient 1
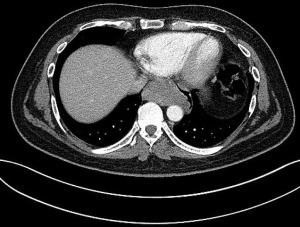
Patient 1 was a 41-year-old man with a history of primary cerebral diffuse large B-cell lymphoma diagnosed 1 year earlier and treated with chemotherapy. At early follow-up CT imaging, a 73 mm tumor located in the subcarinal space was diagnosed (Figure 1).
It showed high fludeoxyglucose (FDG) activity on positron emission tomography-CT (PET/CT). A lymphoma recurrence was suspected and oesophgao-gastro-duodenoscopy (OGD) followed by endoscopic ultrasound (EUS) were performed. It described a tumor located at 32 cm from the incisors and originating from the submucosal plane. EUS-fine-needle aspiration (FNA) was performed but could not establish a diagnosis. A surgical biopsy via video-assisted thoracoscopic surgery (VATS) was performed. Histopathological examination revealed a leiomyoma of the oesophagus. A posterolateral thoracotomy was performed in the 7th intercostal space and the leiomyoma was freed from the oesophagus in its muscular layer. Enucleation was possible without oesophageal resection. Final histopathological examination confirmed the diagnosis of leiomyoma, measuring 82 mm. After an uneventful postoperative stay, the patient was discharged on postoperative day 7.
Patient 2
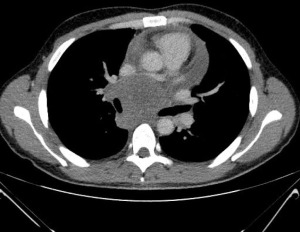
Patient 2 was a 33-year-old male who presented with coughing and a sudden decline in exercise capacity. Transthoracic and transoesophageal echocardiography revealed a large lesion of the middle mediastinum. On axial CT imaging, it measured 84 mm and was located anteriorly to the tracheal carina, compressed cephalad the right pulmonary artery and caudally the left atrium and oesophagus (Figure 2).
EBUS-TBNA was inconclusive and mediastinoscopy was performed in another hospital. Histopathological examination revealed a synovial sarcoma. Staging was completed with CT of the abdomen and did not reveal distant metastases. He underwent 6 cycles of chemotherapy with doxorubicin and ifosfamide. Re-staging chest/abdomen CT showed partial remission. On magnetic resonance imaging (MRI) of the chest/heart, the tumor shrunk down to 2.5 cm × 4 cm × 4 cm and there was no clear evidence of heart invasion.
Posterolateral thoracotomy was performed in the fourth intercostal space. After having freed the tumor from the lung with a lung wedge resection, tumor was sharply freed from the trachea-right main bronchus and superior vena cava. The azygos vein was sacrificed. Dissection was pursued intrapericardially. Evidence of infiltration of bilateral upper and lower veins, left atrium and right atrium at the junction with the inferior vena cava made a complete tumor resection impossible. Debulking was performed. Histopathological assessment confirmed a G2 monophasic synovial sarcoma. Patient was discharged on postoperative day 3. He underwent chemoradiation with 7 cycles of cisplatin and 70.2 Gy. One month later, mediastinal as well as pleuropulmonary progression were identified. Palliative radiotherapy was administered to the pleura (40 Gy) and the mediastinum (40 Gy) followed by maintenance chemotherapy with trofosfamide. Treatment was switched to pazopanib at new progression. The patient died 13 and 8 months after initial diagnosis and surgery, respectively.
Patient 3
Patient 3 was described elsewhere in more details (8).
Briefly, she was a 70-year-old female, who has been suffering from increasing dyspnea for the last 6 months. Axial chest CT imaging showed a 90 mm subcarinal mass with compression of the tracheal carina and oesophagus. An EBUS-TBNA was performed. Cytopathological examination was compatible with a leiomyosarcoma. OGD showed extrinsic oesophageal compression without mucosal infiltration. Staging including FDG PET/CT could exclude distant metastases and a gynaecological evaluation could exclude an extramediastinal primary. Preoperative MRI is shown in Figure 3A.
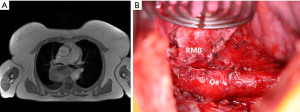
Upfront surgery was favoured over induction chemotherapy. A right posterolateral thoracotomy in the fifth intercostal space was performed. After opening of the mediastinal pleura, the tumor could be freed from the lung, airways, oesophagus and spine. The tumor was removed (Figure 3B) and radical mediastinal lymph node dissection was performed.
After an eventful hospital stay, she was discharged on postoperative day 6. Histopathological examination confirmed a completely resected conventional G2 leiomyosarcoma. All 37 mediastinal lymph nodes were free of tumor. She underwent adjuvant chemotherapy with 5 cycles of dacarbazine and doxorubicin followed by 60 Gy of radiation. Follow-up at 10 months did not show evidence of recurrence.
Patient 4
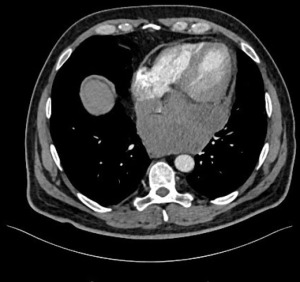
Patient 4 was a 53-year-old male presenting initially with fatigue, sore throat and fever. Later he developed chest pain, weight loss and night sweat. Chest CT revealed a 120 mm tumor on axial imaging, located posterior to the left atrium (Figure 4).
Transoesophageal echocardiography showed compression of the left atrium and ventricle, without hemodynamic relevance. Myocardium was not invaded on heart MRI. Cytopathological examination from EBUS-TBNA showed a round cell sarcoma. He underwent two cycles of doxorubicin and ifosfamide with partial response.
A median sternotomy was performed. The pericardium was opened and intrapericardial adhesions were divided. Right atrium and ventricle were free of tumor. Dissection was continued posteriorly toward the base of the heart. Left atrial invasion by the tumor was suspected. Aorto-bicaval cannulation was performed and cardiopulmonary bypass (CPB) was started. Of note, the inferior vena cava (IVC) was drained from the femoral vein. Tumor resection started at the level of the diaphragmatic pericardium and was continued anteriorly. Left ventriclular infiltration by the tumor was suspected. It was decided to divide the IVC to allow better inspection of the left ventricle. The aorta was crossclamped and cardioplegia was applied. Under full cardiopulmonary bypass the IVC was divided. This maneuver allowed confirmation of left ventricle infiltration. The tumor could be removed without damaging the left ventricle wall. The IVC was reanastomosed and the patient was weaned from CPB. The patient was discharged on postoperative day 7.
Final histopathological assessment confirmed a 4.3 cm undifferentiated round cell sarcoma. The patient underwent additional 4 cycles of doxorubicin and ifosfamide chemotherapy followed by radiation with 66 Gy. Six months postoperative and 9 months after initial diagnosis the patient is well without evidence of tumor progression.
Discussion
Mediastinal lesions are rare and most of them are located in the anterior compartment. In a study including 3,414 healthy individuals who underwent chest CT for medical check-ups, the prevalence of mediastinal tumors was 0.9%, while the prevalence for middle mediastinal tumors was 0.1% (9). Giant middle mediastinal tumors represent a small minority of middle mediastinal tumors, despite the lack of an officially recognized definition for the term “giant”. We suggested a definition based on size measured as the largest lesion diameter on axial imaging from chest CT at diagnosis. “Giant” middle mediastinal lesions were lesions equal or larger than the ninetieth percentile lesion size, namely equal or larger than 73 mm. Interestingly, all four patients who had giant middle mediastinal lesions had tumors of mesenchymal origin, despite the extreme rarity of mesenchymal tumors. Indeed, mediastinal sarcomas represent less than 10% of all mediastinal tumors and account for only 1–2% of all soft tissue sarcomas (10). Our definition for “giant” lesion of the middle mediastinum added diagnostic interest since it could capture exclusively mesenchymal tumors. Despite diverging definitions for giant tumors, results from literature research were in line with our results. The case of a giant middle mediastinal schwannoma originating from the left recurrent nerve and measuring 8 cm on axial CT imaging was reported (11). Cases of angiolipoma, fibrolipoma, lipoma as well as synovial sarcoma were also described as giant primary middle mediastinal tumors (12-15). Conclusions on the relation between histologic entity and tumor size could not be drawn from larger series of primary mediastinal sarcomas due to the lack of precise data on tumor size and compartment location (16,17). The reason why all giant middle mediastinal tumors were of mesenchymal origin is unknown. The specific growth of some mesenchymal tumors, following the path of least resistance and therefore becoming symptomatic later in the disease could play a hypothetic role. In any cases, these results should be confirmed prospectively. Surgical treatment for giant middle mediastinal lesions is challenging due to their size and location. Location in close vicinity to vital structures such as heart and great vessels impose a clear frontier to resectability. Complete resection of primary mediastinal sarcoma is uncommon. In a literature review based on 22 articles including 40 patients, median tumor size was 11 cm and resection was complete in 58% (18). Complete resection was the only factor impacting survival in the univariate analysis, with 5-year survival of 63% and 0% in favour of complete resection (P=0.003). In the largest series of primary mediastinal sarcoma including 976 patients from the National Cancer Database, only 48.9% of patients underwent resection, while the most common treatment was radiation and/or chemotherapy (16). Out of surgically resected patients, microscopic complete (R0) resection was obtained only in 33.8% (16). This is very low compared to primary thoracic sarcomas of other locations (4,19). Histopathologic diagnosis is important in guiding multimodality treatment and planning surgical resection. Specific sarcoma entities are commonly treated preoperatively with induction chemotherapy such as Ewing Sarcoma (20). Preoperative imaging work-up with transoesophageal echo, cardiac MRI or CT may not always give reliable information on heart infiltration by the tumor. Knowledge of the histopathologic diagnosis could refine preoperative imaging interpretation. Specific histopathologic tumor entities behave differently regarding structure infiltration. Benign mesenchymal tumors (lipoma, leiomyoma etc.) as well as liposarcoma do not usually invade adjacent structures and middle mediastinal tumor removal is expected without resection of surrounding structures. Rarely, liposarcoma may invade adjacent structures in case of recurrence or previous surgery. On the other hand, synovial sarcoma, round cell sarcoma or Ewing Sarcomas are highly aggressive tumors that do infiltrate neighbouring structures and complete resection requires en bloc resection of infiltrated surrounding structures. Complete resection and survival rates were probably highly overrated in this review due to publication bias inherent to reviews based on case reports or small case series, where there is a tendency to report long-term survivors after complete tumor resections and underreport patients with suboptimal outcome (18). Common surgical approach for giant middle mediastinal lesions are posterolateral thoracotomy and sternotomy, with or without CPB. Close cooperation of thoracic and cardiac surgeons may be required in this setting. In leiomyoma of the oesophagus, tumor size and location determine the type of surgery. While enucleation is the procedure of choice for smaller leiomyomas, larger leiomyomas (8–10 cm) of the oesophagus may require oesophageal resection and reconstruction (21).
Our study suffers some limitations mainly based on its relatively small sample size and retrospective nature. A selection bias cannot be excluded since only patients who had surgery were included. Due to the rarity of middle mediastinal tumors or primary mediastinal sarcomas strong conclusion on the best management of these tumors is not possible. Second, the prevalence of sarcoma in our cohort may be overestimated due to a recruitment bias related to our sarcoma centre, recruiting patients from the whole country. Third, there was no tumor of cardiac origin in our cohort, since they would have been directly referred to the cardiac surgery department. A larger (multicenter) study also including patients who did not undergo surgery should be performed to validate our results.
Conclusions
Based on our retrospective data analyses of middle mediastinal lesions, the term “giant” could be defined as a mass larger or equal to 73 mm. This definition had a diagnostic impact since it allowed specifically the selection of tumors with mesenchymal origin. This definition may therefore guide diagnostic algorithm and patient management.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://med.amegroups.com/article/view/10.21037/med-22-49/rc
Data Sharing Statement: Available at https://med.amegroups.com/article/view/10.21037/med-22-49/dss
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-22-49/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-22-49/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of University Duisburg-Essen (No. 21-10261-BO) and informed patient consent was waived due to the retrospective analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fujimoto K, Hara M, Tomiyama N, et al. Proposal for a new mediastinal compartment classification of transverse plane images according to the Japanese Association for Research on the Thymus (JART) General Rules for the Study of Mediastinal Tumors. Oncol Rep 2014;31:565-72. [Crossref] [PubMed]
- Whitten CR, Khan S, Munneke GJ, et al. A diagnostic approach to mediastinal abnormalities. Radiographics 2007;27:657-71. [Crossref] [PubMed]
- Carter BW, Tomiyama N, Bhora FY, et al. A modern definition of mediastinal compartments. J Thorac Oncol 2014;9:S97-101. [Crossref] [PubMed]
- Collaud S, Stork T, Schildhaus HU, et al. Multimodality treatment including surgery for primary pulmonary sarcoma: Size does matter. J Surg Oncol 2020;122:506-14. [Crossref] [PubMed]
- Ho JC, Fang P, Cardenas CE, et al. Volumetric assessment of apparent diffusion coefficient predicts outcome following chemoradiation for cervical cancer. Radiother Oncol 2019;135:58-64. [Crossref] [PubMed]
- Li C, Oh SJ, Kim S, et al. Risk factors of survival and surgical treatment for advanced gastric cancer with large tumor size. J Gastrointest Surg 2009;13:881-5. [Crossref] [PubMed]
- Gamliel A, Ziv-Baran T, Siegel RM, et al. Using weight-for-age percentiles to screen for overweight and obese children and adolescents. Prev Med 2015;81:174-9. [Crossref] [PubMed]
- Collaud S, Aigner C. A case report of a giant middle mediastinal leiomyosarcoma. Mediastinum 2022;6:38. [Crossref] [PubMed]
- Kasuga I, Maezawa H, Gamo S, et al. Prevalence of Mediastinal Tumors Using Low-Dose Spiral Computed Tomography in Healthy Population. J Thorac Oncol 2018;13:abstr S605.
- Suster DI, Suster S. Foreword to the Mediastinal Sarcomas Series. Mediastinum 2020;4:29. [Crossref] [PubMed]
- Gueldich M, Hentati A, Chakroun A, et al. Giant cystic schwannoma of the middle mediastinum with cervical extension. Libyan J Med 2015;10:27409. [Crossref] [PubMed]
- Minematsu N, Minato N, Kamohara K, et al. Complete removal of heart-compressing large mediastinal lipoma: a case report. J Cardiothorac Surg 2010;5:48. [Crossref] [PubMed]
- Liu P, Che WC, Ji HJ, et al. A giant infiltrating angiolipoma of the mediastinum: a case report. J Cardiothorac Surg 2016;11:164. [Crossref] [PubMed]
- Hsu JS, Kang WY, Liu GC, et al. Giant fibrolipoma in the mediastinum: an unusual case. Ann Thorac Surg 2005;80:e10-2. [Crossref] [PubMed]
- Rea G, Francesco S, Valente T, et al. Primary mediastinal giant synovial sarcoma: A rare case report. The Egyptian Journal of Radiology & Nuclear Medicine 2014;46:9-12. [Crossref]
- Engelhardt KE, DeCamp MM, Yang AD, et al. Treatment Approaches and Outcomes for Primary Mediastinal Sarcoma: Analysis of 976 Patients. Ann Thorac Surg 2018;106:333-9. [Crossref] [PubMed]
- Paquette M, Truong PT, Hart J, et al. Primary sarcoma of the mediastinum: a report of 16 cases referred to the British Columbia Cancer Agency. J Thorac Oncol 2010;5:898-906. [Crossref] [PubMed]
- Salah S, Salem A. Primary synovial sarcomas of the mediastinum: a systematic review and pooled analysis of the published literature. ISRN Oncol 2014;2014:412527. [Crossref] [PubMed]
- Collaud S, Stork T, Dirksen U, et al. Surgical Treatment for Primary Chest Wall Sarcoma: A Single-Institution Study. J Surg Res 2021;260:149-54. [Crossref] [PubMed]
- Zöllner SK, Amatruda JF, Bauer S, et al. Ewing Sarcoma-Diagnosis, Treatment, Clinical Challenges and Future Perspectives. J Clin Med 2021;10:1685. [Crossref] [PubMed]
- Kandasamy D, Ahamed N, Kannan S, et al. Giant Leiomyoma of the Oesophagus. J Clin Diagn Res 2017;11:PD07-8. [PubMed]
Cite this article as: Collaud S, Stork T, Kaman H, Bauer S, Pöttgen C, Schildhaus HU, Schmack B, Aigner C. Giant middle mediastinal lesions: when tumor size correlates with mesenchymal origin—a retrospective single-center analysis. Mediastinum 2023;7:24.