
A clinical review of spontaneous pneumomediastinum
Introduction
Pneumomediastinum is a rare clinical entity (1). It is defined as air within the mediastinum, and is less often termed mediastinal emphysema. The mediastinum is defined as the visceral space bounded laterally by the parietal pleura, superiorly by the thoracic outlet, inferiorly by the diaphragm, and anteriorly by the sternum, and posteriorly by the thoracic vertebral column. Contained within are the heart, tracheobronchial tree, lung, and esophagus. When air escapes from these organs or structures into the mediastinum, this is termed pneumomediastinum. Symptoms from pneumomediastinum most commonly involve chest pain, and may also include dyspnea, neck swelling, cervical pain, dysphagia, odynophagia, and dysphonia (2,3). Pneumomediastinum may be accompanied by pneumothorax, subcutaneous emphysema, or rarely, pneumorrachis, or air within the spinal canal (4).
Background
Pneumomediastinum is commonly classified into two categories: spontaneous pneumomediastinum and secondary pneumomediastinum. Spontaneous pneumomediastinum occurs in otherwise healthy subjects without obvious causative factor. Spontaneous pneumomediastinum, however, has predisposing factors, such as smoking and recreational drug use (5,6). Secondary pneumomediastinum is due to an identifiable causal factor. These may include iatrogenic causes, such as intubation, thoracic surgery, thoracostomy tube placement, or central vascular access, or may be due to traumatic injuries (7). Other causes of secondary pneumomediastinum include asthma, air trapping, bronchiectasis, child birth, chronic obstructive pulmonary disease (COPD), coronavirus disease 2019 (COVID-19), inhalation of toxic fumes, interstitial lung disease, malignancy, marijuana use, mechanical ventilation, and physical activity (8-15). Exacerbations in respiratory diseases or infections often lead to pneumomediastinum when exacerbations with excessive coughing cause an increase in endopulmonary pressure (7). Similarly, child birth, scuba-diving, retching, and certain types of physical activity may cause increased endopulmonary pressure, leading to pneumomediastinum (7).
The suspected pathophysiology for spontaneous pneumomediastinum is commonly termed the “Macklin effect”. Evidence for this arises from Macklin in the 1944 study conducted on cats (16). Increased endopulmonary pressure leads to alveolar rupture; air migrates through the peribronchial and perivascular sheathes towards the mediastinum (16). Increased alveolar pressure may result directly, from coughing or inhalation injury, or indirectly from forceful retching and vomiting leading to increased alveolar pressure, and thus alveolar rupture and pneumomediastinum (17).
Rationale and knowledge gap
Limited evidence exists guiding clinical decision making and treatment for pneumomediastinum. Specifically, no randomized clinical trials exist to guide pneumomediastinum management in either spontaneous or secondary causes of pneumomediastinum. The highest level of evidence guiding pneumomediastinum treatment comes from various case series and reports. However, due to the various etiologies of pneumomediastinum, these may be difficult to apply broadly, and need to be considered on a case-by-case basis (8).
Objective
This review aims to gather the current evidence guiding isolated pneumomediastinum (pneumomediastinum without associated effusion) diagnosis and management, with respect to two clinical scenarios: (I) presentations with low suspicion for esophageal rupture, such as patients with excessive coughing; and (II) presentation with higher concern for esophageal rupture, such as vomiting or retching.
Overview of pneumomediastinum
Pneumomediastinum is a rare clinical entity with incidence of 1/25,000 in age 5–34 years (18). In younger patients, spontaneous pneumomediastinum is more common, with an incidence of 1/14,000 (19). However, some posit that pneumomediastinum is underdiagnosed, due to either patients refraining from seeking medical care, misdiagnosis as musculoskeletal pain, or missed pneumomediastinum on chest X-ray (CXR) (20,21). A male predominance is noted, occurring in a male-to-female ratio of 3.6:1 (22). The prototypical patient with pneumomediastinum exhibits pre-existing lung disease, such as asthma, and is a male of tall and lean habitus (3,23).
Patient presentation: history and physical exam
Patients with pneumomediastinum typically present with the chief complaint of acute retrosternal chest pain radiating to the neck or back (9). This is present in about 60–100% of patients (9). They may also present with dyspnea (75%), neck swelling, cervical pain (36%), dysphagia, odynophagia, and dysphonia (2,3,9). In order to ascertain the causative factor, the patient history should include inciting factors, such as episodes of coughing or episodes of forceful retching or vomiting. For example, an asthma exacerbation may be a precipitating cause of a coughing episode. Alternatively, a patient with hyperemesis gravidarum may present after significant vomiting (7). Predisposing factors, such as respiratory diseases, inhalation injury, smoking, and recreational drug use increase suspicion for pneumomediastinum (7,8). Commonly, the causative factor is unknown, and pneumomediastinum is discovered incidentally (9,10).
Upon exam, patients may present with tachycardia and tachypnea (7). A notable physical exam finding in pneumomediastinum is the Hamman’s sign. Hamman’s sign, also known as Hamman’s crunch, is the presence of a “crunch” synchronous with the heart beat on cardiac auscultation (24). Subcutaneous emphysema (70%) may be present as well (10,12).
Diagnostic imaging and other adjuncts
Diagnosis is commonly made on anterior CXR; CXR alone may be diagnostic in 73–90% of patients (3,10,25). Common radiologic signs include the “continuous diaphragm sign” caused by posterior pericardial air, “extra-pleural air sign” caused by air extending between the parietal pleural laterally towards the diaphragm, and the “thymic sail sign” caused by air elevating the thymus. Others include shining bundles surrounding the mediastinal organs and mediastinal pleura separating from the cardiac edge (17,26-28).
Whether CXR alone is sufficient for diagnosis of pneumomediastinum remains contentious. As up to 30% of patients with pneumomediastinum present with normal CXR, it is suggested that inclusion of chest computed tomography (CT) be performed if there is suspicion for pneumomediastinum despite normal CXR, to differentiate between pneumopericardium or subcutaneous emphysema, or to investigate for causes of pneumomediastinum (17,21,23,29). Chest CT can be beneficial in detecting injury to the tracheobronchial system, pneumothorax [present in up to 40% of patients (3)], or esophageal perforation, each of which may affect management (25).
Other minimally invasive adjuncts in diagnosis include bedside thoracic ultrasound, which may aid in more rapid recognition of pneumomediastinum as a cause of acute onset chest pain. Findings on thoracic ultrasound include poor visualization of the heart, diffuse A lines, and normal visualization of the heart from the subxiphoid view (12,30). Laboratory and electrocardiogram (ECG) findings are non-diagnostic and non-specific; laboratory findings may demonstrate leukocytosis or increased C-reactive protein (CRP) while ECG findings may mimic acute pericarditis (31,32). Echocardiography may be used in case of suspicion for pneumopericardium.
Diagnosis and management per etiology
As previously described, the etiology of pneumomediastinum is often challenging to ascertain. The most likely cause of pneumomediastinum is due to alveolar rupture as a result from increased Valsalva pressure from prolonged coughing or retching (33). A more detailed patient history can help narrow the range of possible etiologies of pneumomediastinum, and target diagnostic workup to rule out the most likely associated injury.
Patient with coughing: lower concern for esophageal perforation
Diagnosis
The patient presentation that may raise concern for airway or lung etiology of pneumomediastinum usually demonstrates a significant episode of coughing (8,34). Past medical history that may be associated includes lung-related pathology, i.e., asthma, COPD, bronchiectasis, and interstitial lung disease (7). Clear causes of airway or lung injury, such as mechanical ventilation, intubation, or other non-iatrogenic trauma raise more direct concern for airway or lung etiology of pneumomediastinum.
As standard for pneumomediastinum, one would begin with CXR. If CXR is non-diagnostic, but suspicion remains high for pneumomediastinum, CT chest may be obtained (Figure 1) (7,25).
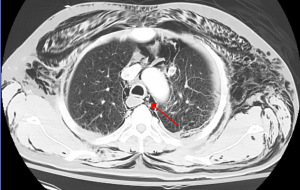
Further diagnostic maneuvers, such as bronchoscopy, laryngoscopy, esophagoscopy, or esophagogram need not be routinely performed, but selectively performed based on clinical suspicion (3,7,25). If clinical history suggests likely airway injury, as in the case of traumatic injury, bronchoscopy should be performed. Clinical instability as defined by unstable vital signs, would also necessitate more aggressive workup, including repeat CT chest, bronchoscopy to rule out missed air leak and esophagram, and/or esophagoscopy to rule out esophageal injury (7,9,10).
However, if the patient is clinically stable, as determined by normal vital signs and laboratory values, and low suspicion for bronchial or esophageal injury exists, routine bronchoscopy, esophagoscopy, and esophagogram are unlikely to provide clinical benefit. In Song et al., 45 patients with spontaneous pneumomediastinum were studied, 90% underwent esophagogram, 31% bronchoscopy, and 2.2% endoscopy, and no injury was found (25). Similar findings have suggested futility of routine extensive workups, and favor a more selective approach to the workup of pneumomediastinum (10,34).
Management
Management of spontaneous pneumomediastinum largely consists of supportive care, which has historically been defined by oxygen inhalation therapy, pain control, and bed rest (3,9,10,18). The theory behind oxygen inhalational therapy is to increase the diffusion pressure of nitrogen in the interstitium and promote free air absorption in the mediastinum (25,35). Consensus as to admission, length of admission, diet restriction, and antibiotic control has not yet been reached, likely due to limited controlled trials. Though earlier studies suggest hospitalization for approximately 2–5 days, this is based on retrospective data that collects the average length of stay for spontaneous pneumomediastinum. The data lacks granularity to clarify the reason for admission and thus should not be taken as prescriptive (9,10). Hospital admission and length of stay may be minimized in uncomplicated spontaneous pneumomediastinum without compromising patient safety (3,25,31). A clinical judgment for admission versus observation then discharge in each clinical scenario is reasonable. Patients with pain, nausea, or other ongoing symptoms may warrant admission (35,36). During admission, supplemental oxygen may be given as clinically warranted.
Diet restriction and prophylactic antibiotic usage for prevention of mediastinitis is advocated for by some (18,31). However, more recent studies note a lack of evidence for true benefit; in the patient with lower risk features, such as age less than 40 years, presentation with cough, normal white blood cell (WBC) count, and no evidence of pleural effusion, suspicion for esophageal injury is low, and recommendation is instead to advance diet as tolerate and avoid prophylactic antibiotics (37).
If concomitant significant pneumothorax is noted, thoracostomy tube placement may be appropriate. However, this is generally a low percentage of patients (6%), and may be more likely to present in patients who are mechanically ventilated or have associated pulmonary diseases where pneumothoraces are more likely to develop (3,10,23).
In cases that pneumomediastinum progresses to airway compression, video-assisted thoracoscopy (VATS) or thoracotomy may be necessitated for decompression (38). Additionally, tension pneumopericardium may develop, leading to cardiac tamponade, requiring surgical intervention by VATS or pericardial window (9).
Rarely, a tracheal or bronchial injury is found (3,9,10,18,21). In the case of tracheal injury, operative management is usually necessitated. However, in bronchial injuries stenting is increasingly used, particularly in cases of iatrogenic injury (39).
Patient with vomiting/retching: higher suspicion for esophageal perforation
Diagnosis
Pneumomediastinum may also arise in patients with severe and repeated vomiting and retching. This may be due to excessive alcohol ingestion, recreational drug use, hyperemesis gravidarum, and ingestion of caustic substances (7,34). The history of repeated retching could lead to alveolar rupture via Macklin effect (7,16,33).
Given this difference in patient history, the initial CXR should be immediately followed by CT of the chest and abdomen (3,25,34). If high-risk features such as age greater than 40 years, abdominal pain, leukocytosis, CT findings of pleural effusion, or pneumoperitoneum, one should maintain an even higher concern for esophageal perforation and proceed with either contrast esophagram or CT esophagogram with or without esophagoscopy immediately thereafter (34,40,41). For contrast esophagram, it is recommended to start with water-soluble esophagram, and then confirm with barium esophagram to minimize contrast extravasation into the mediastinal or pleural space. A summary of the diagnostic algorithm for both pathways is provided in Figure 2.
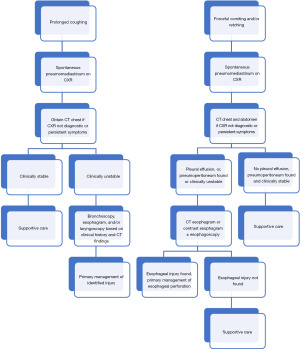
Management
In the absence of diagnostic evidence of esophageal injury, management of pneumomediastinum in this scenario is similar to that above. The main treatment is supportive care, including bowel rest and pain control (3,9,10,25,42). Once symptoms of pneumomediastinum are resolved, patients can be started on a liquid diet and advanced as tolerated. In the absence of evidence for esophageal perforation, prophylactic antibiotics are not required (25,34).
Long-term follow-up of pneumomediastinum
Pneumomediastinum is self-resolving through air resorption in the mediastinal tissues, though in some cases may be notable on imaging for up to 6 months (9,10). Recurrence of pneumomediastinum is exceedingly rare, approximately 1% (31,43). However, a recent by Kumeda et al. in 2023 demonstrates a surprisingly high recurrence rate of 17% (44). Though it is unknown if this particular result is secondary to a small sample size or if the true recurrence rate is higher than previously thought, identifying patients with risk factors for recurrence, such as asthma, is essential (45). Given such low rates of pneumomediastinum recurrence in the majority of studies thus far, long-term follow-up may not be a universal requirement, but evaluated on a case-by-case basis.
Pneumomediastinum in children
Management of spontaneous pneumomediastinum in children is largely similar to that of adult spontaneous pneumomediastinum (19). Spontaneous pneumomediastinum is rarer in children than adults. However, incidence is challenging to ascertain and is likely underdiagnosed (46,47). Asthma remains the most frequent comorbidity in children, with approximately 22% of patients with pneumomediastinum presenting with this comorbidity (47). Secondary spontaneous pneumomediastinum is most common caused by asthma exacerbation, and may also be caused by pneumonia, lower respiratory tract infections, or choking. In a study of 87 pediatric patients at a tertiary children’s facility in Taiwan, all patients younger than 6 years had secondary spontaneous pneumomediastinum, versus 60.6% of patients older than 6 years. Diagnosis remains similar, with CXR followed by chest CT only if diagnosis is unclear and suspicion for pneumomediastinum remains high. Further diagnostic testing beyond CXR (after establishing diagnosis) is unlikely to affect management in clinically stable children (48,49).
Routine further invasive testing, such as esophagram and laryngoscopy are unlikely to yield diagnostic information (48). Thus, a difference in diagnostic workup is that for clinically well-appearing children, unless suspicion for associated airway, lung, or esophageal injury is high, extended diagnostic workup including bronchoscopy, laryngoscopy, or esophagram is unwarranted (49). Patients may be managed conservatively with clinical observation, rest, pain control, and oxygen therapy and will likely have spontaneous resolution (47,50).
A key clinical difference is that in the absence of known respiratory disease (i.e., asthma), spontaneous pneumomediastinum in children without known cause should prompt outpatient pulmonary function testing (PFTs) to investigate whether the patient has asthma or other respiratory disease (7,19).
Future spontaneous pneumomediastinum guidelines
Presented in this article is a clinical review of pneumomediastinum and suggested diagnostic and therapeutic pathways based on suspected etiology of pneumomediastinum. The strengths of this article include gathering available evidence arising from a thorough literature review on PubMed for spontaneous pneumomediastinum. Primary evidence regarding spontaneous pneumomediastinum diagnosis and management is a combination of various case reports, case series, and retrospective cohort studies. By combining this literature as well as the author’s own diagnostic and management algorithm from a high-volume thoracic surgery regionalized center, a standardized, easy-to-follow algorithm is presented. However, limitations due to the lack of prospective, controlled trial evidence limits the strength of the recommendations for mandated diagnostic and treatment algorithms in this disease process.
Conclusions
Pneumomediastinum is a rare condition with multiple etiologies, and often no known etiology. Spontaneous pneumomediastinum may be diagnosed with limited workup, such as CXR and/or CT. Secondary pneumomediastinum diagnosis and treatment may be streamlined based on suspected etiology. Clues to etiology often come from patient history and presenting symptoms. Although evidence from randomized controlled trials are lacking, case series suggest that pneumomediastinum is generally benign and self-limited if no associated injury is identified. Management is largely supportive care and symptomatic management. Recurrences are rare, and thus limited follow up is necessary. Higher level evidence is needed to solidify treatment algorithms for pneumomediastinum in the future, which may further promote conservative management and limit unnecessary diagnostic procedures.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-23-25/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-23-25/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Al-Mufarrej F, Badar J, Gharagozloo F, et al. Spontaneous pneumomediastinum: diagnostic and therapeutic interventions. J Cardiothorac Surg 2008;3:59. [Crossref] [PubMed]
- Alemu BN, Yeheyis ET, Tiruneh AG. Spontaneous primary pneumomediastinum: is it always benign? J Med Case Rep 2021;15:157. [Crossref] [PubMed]
- Iyer VN, Joshi AY, Ryu JH. Spontaneous pneumomediastinum: analysis of 62 consecutive adult patients. Mayo Clin Proc 2009;84:417-21. [Crossref] [PubMed]
- Song Y, Tu L, Wu J. Pneumorrhachis with spontaneous pneumomediastinum and subcutaneous emphysema. Intern Med 2009;48:1713-4. [Crossref] [PubMed]
- De La Cruz Morón I, Reyes Núñez N, Rojas Box JL. Neumomediastino espontáneo en un consumidor de cocaína. Arch Bronconeumol 2000;36:231. [Crossref] [PubMed]
- Aroesty DJ, Stanley RB, Crockett DM. Pneumomediastinum and cervical emphysema from the inhalation of "free based" cocaine: report of three cases. Otolaryngol Head Neck Surg 1986;94:372-4. [Crossref] [PubMed]
- Kouritas VK, Papagiannopoulos K, Lazaridis G, et al. Pneumomediastinum. J Thorac Dis 2015;7:S44-9. [PubMed]
- Kobashi Y, Okimoto N, Matsushima T, et al. Comparative study of mediastinal emphysema as determined by etiology. Intern Med 2002;41:277-82. [Crossref] [PubMed]
- Macia I, Moya J, Ramos R, et al. Spontaneous pneumomediastinum: 41 cases. Eur J Cardiothorac Surg 2007;31:1110-4. [Crossref] [PubMed]
- Caceres M, Ali SZ, Braud R, et al. Spontaneous pneumomediastinum: a comparative study and review of the literature. Ann Thorac Surg 2008;86:962-6. [Crossref] [PubMed]
- Chu CM, Leung YY, Hui JY, et al. Spontaneous pneumomediastinum in patients with severe acute respiratory syndrome. Eur Respir J 2004;23:802-4. [Crossref] [PubMed]
- Russo A, Del Vecchio C, Zaottini A, et al. Role of emergency thoracic ultrasonography in spontaneous pneumomediastinum. Two case report. G Chir 2012;33:285-96. [PubMed]
- Raley JC, Andrews JI. Spontaneous pneumomediastinum presenting as jaw pain during labor. Obstet Gynecol 2001;98:904-6. [PubMed]
- Reyes S, Roche B, Kazzaz F, et al. Pneumothorax and pneumomediastinum in COVID-19: A case series. Am J Med Sci 2022;363:548-51. [Crossref] [PubMed]
- Yu I, Tung K, Dugan R, et al. Dedicated esophageal imaging may be unnecessary in marijuana-associated spontaneous pneumomediastinum: Findings from a retrospective cohort study. Front Surg 2023;10:1043729. [Crossref] [PubMed]
- Macklin MT, Macklin CC. Malignant interstitial emphysema of the lungs and mediastinum as an important occult complication in many respiratory diseases and other conditions: an interpretation of the clinical literature in the light of laboratory experiment. Medicine 1944;23:281-358. [Crossref]
- Zylak CM, Standen JR, Barnes GR, et al. Pneumomediastinum revisited. Radiographics 2000;20:1043-57. [Crossref] [PubMed]
- Jougon JB, Ballester M, Delcambre F, et al. Assessment of spontaneous pneumomediastinum: experience with 12 patients. Ann Thorac Surg 2003;75:1711-4. [Crossref] [PubMed]
- Chalumeau M, Le Clainche L, Sayeg N, et al. Spontaneous pneumomediastinum in children. Pediatr Pulmonol 2001;31:67-75. [Crossref] [PubMed]
- Sahni S, Verma S, Grullon J, et al. Spontaneous pneumomediastinum: time for consensus. N Am J Med Sci 2013;5:460-4. [Crossref] [PubMed]
- Kaneki T, Kubo K, Kawashima A, et al. Spontaneous pneumomediastinum in 33 patients: yield of chest computed tomography for the diagnosis of the mild type. Respiration 2000;67:408-11. [Crossref] [PubMed]
- Ryoo JY. Clinical analysis of spontaneous pneumomediastinum. Tuberc Respir Dis (Seoul) 2012;73:169-73. [Crossref] [PubMed]
- Mondello B, Pavia R, Ruggeri P, et al. Spontaneous pneumomediastinum: experience in 18 adult patients. Lung 2007;185:9-14. [Crossref] [PubMed]
- Alexandre AR, Marto NF, Raimundo P. Hamman's crunch: a forgotten clue to the diagnosis of spontaneous pneumomediastinum. BMJ Case Rep 2018;2018:bcr2018225099. [Crossref] [PubMed]
- Song IH, Lee SY, Lee SJ, et al. Diagnosis and treatment of spontaneous pneumomediastinum: experience at a single institution for 10 years. Gen Thorac Cardiovasc Surg 2017;65:280-4. [Crossref] [PubMed]
- Bejvan SM, Godwin JD. Pneumomediastinum: old signs and new signs. AJR Am J Roentgenol 1996;166:1041-8. [Crossref] [PubMed]
- Levin B. The continuous diaphragm sign. A newly-recognized sign of pneumomediastinum. Clin Radiol 1973;24:337-8. [Crossref] [PubMed]
- Lillard RL, Allen RP. The extrapleural air sign in pneumomediastinum. Radiology 1965;85:1093-8. [Crossref] [PubMed]
- Ho AS, Ahmed A, Huang JS, et al. Multidetector computed tomography of spontaneous versus secondary pneumomediastinum in 89 patients: can multidetector computed tomography be used to reliably distinguish between the 2 entities? J Thorac Imaging 2012;27:85-92. [Crossref] [PubMed]
- Zachariah S, Gharahbaghian L, Perera P, et al. Spontaneous pneumomediastinum on bedside ultrasound: case report and review of the literature. West J Emerg Med 2015;16:321-4. [Crossref] [PubMed]
- Koullias GJ, Korkolis DP, Wang XJ, et al. Current assessment and management of spontaneous pneumomediastinum: experience in 24 adult patients. Eur J Cardiothorac Surg 2004;25:852-5. [Crossref] [PubMed]
- Chaudhary H, Yousaf Z, Nasir U, et al. Spontaneous pneumomediastinum mimicking acute pericarditis. Clin Case Rep 2021;9:e05156. [Crossref] [PubMed]
- Sakai M, Murayama S, Gibo M, et al. Frequent cause of the Macklin effect in spontaneous pneumomediastinum: demonstration by multidetector-row computed tomography. J Comput Assist Tomogr 2006;30:92-4. [Crossref] [PubMed]
- Bakhos CT, Pupovac SS, Ata A, et al. Spontaneous pneumomediastinum: an extensive workup is not required. J Am Coll Surg 2014;219:713-7. [Crossref] [PubMed]
- Kim KS, Jeon HW, Moon Y, et al. Clinical experience of spontaneous pneumomediastinum: diagnosis and treatment. J Thorac Dis 2015;7:1817-24. [PubMed]
- Morgan CT, Maloney JD, Decamp MM, et al. A narrative review of primary spontaneous pneumomediastinum: a poorly understood and resource-intensive problem. J Thorac Dis 2021;13:3721-30. [Crossref] [PubMed]
- Morgan CT, Kanne JP, Lewis EE, et al. One hundred cases of primary spontaneous pneumomediastinum: leukocytosis is common, pleural effusions and age over 40 are rare. J Thorac Dis 2023;15:1155-62. [Crossref] [PubMed]
- Perna V, Vilà E, Guelbenzu JJ, et al. Pneumomediastinum: is this really a benign entity? When it can be considered as spontaneous? Our experience in 47 adult patients. Eur J Cardiothorac Surg 2010;37:573-5. [Crossref] [PubMed]
- Ajith Kumar AK, Anjum F. Tracheobronchial Tear. In: StatPearls. Treasure Island: StatPearls Publishing; 2023.
- Wu CH, Chen CM, Chen CC, et al. Esophagography after pneumomediastinum without CT findings of esophageal perforation: is it necessary? AJR Am J Roentgenol 2013;201:977-84. [Crossref] [PubMed]
- Whyte RI. Boerhaave's syndrome. N Engl J Med 2001;344:139. [PubMed]
- Takada K, Matsumoto S, Hiramatsu T, et al. Management of spontaneous pneumomediastinum based on clinical experience of 25 cases. Respir Med 2008;102:1329-34. [Crossref] [PubMed]
- Gerazounis M, Athanassiadi K, Kalantzi N, et al. Spontaneous pneumomediastinum: a rare benign entity. J Thorac Cardiovasc Surg 2003;126:774-6. [Crossref] [PubMed]
- Kumeda H, Saito G, Eguchi T, et al. Clinical features of recurrent spontaneous pneumomediastinum. J Thorac Dis 2023;15:462-71. [Crossref] [PubMed]
- Morgan CT. Putting on airs again: new insights and questions about spontaneous pneumomediastinum recurrence. J Thorac Dis 2023;15:2890-2. [Crossref] [PubMed]
- Versteegh FG, Broeders IA. Spontaneous pneumomediastinum in children. Eur J Pediatr 1991;150:304-7. [Crossref] [PubMed]
- Gasser CR, Pellaton R, Rochat CP. Pediatric Spontaneous Pneumomediastinum: Narrative Literature Review. Pediatr Emerg Care 2017;33:370-4. [Crossref] [PubMed]
- Noorbakhsh KA, Williams AE, Langham JJW, et al. Management and Outcomes of Spontaneous Pneumomediastinum in Children. Pediatr Emerg Care 2021;37:e1051-6. [Crossref] [PubMed]
- Fitzwater JW, Silva NN, Knight CG, et al. Management of spontaneous pneumomediastinum in children. J Pediatr Surg 2015;50:983-6. [Crossref] [PubMed]
- Bullaro FM, Bartoletti SC. Spontaneous pneumomediastinum in children: a literature review. Pediatr Emerg Care 2007;23:28-30. [Crossref] [PubMed]
Cite this article as: Susai CJ, Banks KC, Alcasid NJ, Velotta JB. A clinical review of spontaneous pneumomediastinum. Mediastinum 2024;8:4.


