Pneumomediastinum and pneumothorax in acute respiratory distress syndrome (ARDS) patients: a narrative review
Introduction
Acute respiratory distress syndrome (ARDS) is a critical and life-threatening medical condition characterized by the rapid onset of noncardiogenic pulmonary edema, resulting in severe respiratory failure. This complex disorder encompasses many etiologies, with bacterial and viral pneumonia as the most prevalent causes of ARDS. In addition to pulmonary origins, ARDS can also arise from extrapulmonary sepsis, aspiration of gastric contents, severe trauma accompanied by shock, and, less frequently, pancreatitis and drug reactions (1-5). In 1994, The American-European Consensus Conference (AECC) recommended simplified criteria for the diagnosis of ARDS (6), and the severity was further categorized into mild, moderate, and severe based on the degree of hypoxemia, radiographic severity, respiratory system compliance, positive end-expiratory pressure (PEEP), and corrected expired volume per minute in 2012 (7); Despite the heterogeneity of ARDS, significant progress has been made in understanding its pathogenesis through laboratory and clinical studies. Notably, two key pillars of the treatment approach, namely lung-protective ventilation, and fluid-conservative management, have contributed to reducing mortality and morbidity associated with the syndrome. However, the emergence of the coronavirus disease 2019 (COVID-19) pandemic in 2019 presented new challenges, leading to an increased incidence of ARDS and barotrauma, such as pneumothorax (PNX) and pneumomediastinum (PMD) when air collection occurs in the pleural space and mediastinum respectively in patients receiving invasive mechanical ventilation due to alveolar damage secondary to high airway pressure. This review addresses several pertinent issues in ARDS, focusing on providing insights into PNX and PMD among ARDS patients. By examining these specific aspects, we aim to enhance our understanding of the disease and potentially identify strategies for its management and prevention. We present this article in accordance with the Narrative Review reporting checklist (available at https://med.amegroups.com/article/view/10.21037/med-23-39/rc).
Methods
This review summarizes the clinical presentation and management of patients with ARDS complicated by PMD or PNX. We searched PubMed, MEDLINE, and Google Scholar databases for articles on PNX and PMD in ARDS. We used the MeSH terms “PNX”, “PMD”, “Barotrauma”, “Acute respiratory distress syndrome”, and “ARDS”. Studies reporting patient demographics, clinical presentations, and management of PNX and PMD in ARDS patients were included in this review. Publications, including clinical trials, cohort studies, and case-control studies published before July 2023, were included. In addition, the bibliography of selected articles was examined for further studies. We included only studies published in English and excluded articles that included opinions, letters, abstracts, and preprints yet to undergo peer reviews. The search strategy summary is listed below (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | 5th July 2023 |
| Databases and other sources searched | PubMed, MEDLINE, and Google Scholar |
| Search terms used | We used the MeSH terms “PNX”, “PMD”, “Barotrauma”, “Acute respiratory distress syndrome”, and “ARDS” |
| Timeframe | Any publication prior to July 2023 |
| Inclusion & exclusion criteria | Inclusion: clinical trials, cohort studies, and case-control studies published before July 2023, articles in English. Exclusion: articles that included opinions, letters, abstracts, and preprints yet to undergo peer reviews |
| Selection process | The articles were reviewed by authors independently and articles met the inclusion criteria were imported into a reference manager software and was cross-reviewed prior to inclusion |
| Additional considerations | Bibliography of selected articles were examined for further studies |
ARDS
Definitions
ARDS is characterized by the sudden onset of acute hypoxic respiratory failure. Diffuse bilateral pulmonary infiltrates on imaging mark this condition. The definition of ARDS has been modified several times over the past few decades (Table 2). Initially identified as a distinct entity in 1967 by Ashbaugh et al. (8), the definition of ARDS was refined and expanded in 1988 by Murray et al. (9). The globally accepted definition was established during an AECC in 1994 (6).
Table 2
| Parameters | Ashbaugh et al., 1967 (8) | Lung injury score, 1988 (9) | AECC (6) | Berlin criteria (7) | Kigali modification of Berlin score (10) | New global definition, 2023 (11) |
|---|---|---|---|---|---|---|
| Timing | No specific duration of onset was described | No specific duration of onset was described | Acute onset | Within 1 week if a known clinical insult or respiratory symptoms | Within 1 week if a known clinical insult or respiratory symptoms | Within 1 week if a known clinical insult or respiratory symptoms |
| Oxygenation | No particular level of oxygen was described | PaO2/FiO2 225–299: 1 point; PaO2/FiO2 175–224: 2 points; PaO2/FiO2 100–174: 3 points; PaO2/FiO2 <100: 4 points | PaO2/FiO2 ≤300 mmHg | PaO2/FiO2 ≤300 mmHg | SpO2/FiO2 ≤315 | PaO2/FiO2 300 mmHg or SpO2/FiO2 315 (if SpO2 97%) on HFNO with a flow of 30 liters per minute or NIV/CPAP with at least 5 cmH2O expiratory pressure |
| Imaging | Not included in the definition | Points based on the presence of consolidation in different quadrants | Bilateral infiltrates observed on frontal chest radiograph | Bilateral opacities not fully explained by effusions, lobar/lung collapse, or nodules by chest radiograph or CT | Bilateral opacities not fully explained by effusions, lobar/lung collapse, or nodules by chest radiograph or ultrasound | Bilateral opacities on chest radiograph and computed tomography, or bilateral B lines and/or consolidations by ultrasound, not fully explained by effusions, atelectasis, or nodules/masses |
| PEEP | Not included in the definition | Points based on the level of PEEP required | Not included in the definition | Minimum 5 cmH2O PEEP required by invasive mechanical ventilation | No PEEP requirement (due to scarcity of ventilators in resource poor setting) | Based on resource setting; 5 cmH2O in resource rich setting and no PEEP requirement in resource variable setting |
| Pulmonary compliance | Defined as loss of pulmonary compliance but no prespecified cutoff | Points based on the level of pulmonary compliance | Not included in the definition | Not included in the definition | Not included in the definition | Not included in the definition |
| PAWP | Not included in the definition | Not included in the definition | PAWP ≤18 mmHg when measured or no clinical evidence of left atrial hypertension | Not included in the definition | Not included in the definition | Not included in the definition |
| Comments | Characterized by the presence of tachypnea, hypoxia, and loss of compliance after a variety of stimuli | No lung injury: 0 points; mild to moderate lung injury: 0.1 to 2.5 points; severe lung injury: >2.5 points | Not included in the definition | Not included in the definition | Was postulated due to challenges faced in resource poor setting | Not included in the definition |
ARDS, acute respiratory distress syndrome; AECC, American-European Consensus Conference; PaO2, partial pressure of oxygen in artery; FiO2, fraction of inspired oxygen; SpO2, saturation of peripheral oxygen; HFNO, high-flow nasal oxygen; NIV, non-invasive ventilation; CPAP, continuous positive airway pressure; CT, computed tomography; PEEP, positive end-expiratory pressure; PAWP, pulmonary arterial wedge pressure.
The AECC definition of ARDS or acute lung injury (ALI) defined hypoxemia based on the PaO2/FiO2 ratio ≤300 mmHg. This criterion did not take into account the level of PEEP. The reasoning behind this decision was that PEEP can have variable effects on pulmonary shunt fraction, and its response is time-dependent and individualized. Therefore, the oxygenation criteria for ARDS did not include PEEP as a factor (6).
Criteria suggestive of ALI/ARDS per AECC definition:
- Acute onset;
- PaO2/FiO2 ≤300 mmHg (for ALI); PaO2/FiO2 ≤200 mmHg (for ARDS);
- Pulmonary capillary wedge pressure (PCWP) ≤18 mmHg or no clinical evidence of left atrial hypertension.
These criteria were globally accepted and were crucial in guiding clinical practice, particularly in the landmark ARDSnet ARMA trial that advocated adopting protective lung ventilation strategies (12). However, clinicians have faced challenges when applying this definition, particularly in assessing the severity of hypoxemia when PEEP is implemented. Applying PEEP can influence the PaO2/FiO2 ratio, making it difficult to accurately gauge the extent of hypoxemia (13). However, despite the limitations, a reasonable correlation has been demonstrated between the AECC definition and ARDS autopsy findings with a sensitivity of 75–83% and specificity of 51–84% (14,15).
In response to the challenges and limitations of the AECC definition, the Berlin definition of ARDS was introduced in 2012 as an updated framework (7). The Berlin definition abandoned the term “acute lung injury” and focused solely on ARDS. It refined the “acute” onset time frame, setting it within seven days of worsening respiratory symptoms. One notable change in the Berlin definition was replacing the PCWP criterion with a less invasive measures, such as echocardiography, to exclude cardiac-related causes of bilateral infiltrates. The Berlin definition also introduced three severity categories for ARDS based on the degree of hypoxemia, as measured by the PaO2/FiO2 ratio. Importantly, this ratio was now related to a minimum PEEP requirement for adequate oxygenation. This addition recognized the impact of PEEP on oxygenation and allowed for a more comprehensive assessment of ARDS severity (Table 3).
Table 3
| Component | Definition |
|---|---|
| Timing | Within one week of known clinical insult or new or worsening respiratory symptoms |
| Chest imaginga | Bilateral opacities-not fully explained by effusions, lobar/lung collapse, or nodules |
| Origin of edema | Respiratory failure not fully explained by cardiac failure or fluid overload. Need objective assessment to exclude hydrostatic edema if no risk factor present |
| Oxygenationb | |
| Mild | 200 mmHg < PaO2/FiO2 ≤300 mmHg with PEEP or CPAP ≥5 cmH2Oc |
| Moderate | 100 mmHg < PaO2/FiO2 ≤200 mmHg with PEEP ≥5 cmH2O |
| Severe | PaO2/FiO2 ≤100 mmHg with PEEP ≥5 cmH2O |
a, chest radiograph or computed tomography scan; b, if altitude is more than 1,000 m, the correction factor should be calculated as: [PaO2/FiO2 × (barometric pressure/760)]; c, this may be delivered noninvasively in the mild ARDS group. ARDS, acute respiratory distress syndrome; PaO2, partial pressure of oxygen in artery; FiO2, fraction of inspired oxygen; PEEP, positive end-expiratory pressure; CPAP, continuous positive airway pressure.
Since the 2012 Berlin definition, several developments listed below support the need for a revised global definition of ARDS:
- Increased high-flow nasal cannula (HFNC) use for acute respiratory failure: HFNC has gained popularity as a non-invasive respiratory support modality. However, some patients receiving HFNC may not meet the criteria outlined in the Berlin definition for ARDS. This has raised questions about including these patients in the ARDS diagnostic framework and the need to reassess the definition accordingly.
- Validation of SpO2/FiO2 by pulse oximetry: The SpO2/FiO2 ratio measured by pulse oximetry is a reliable and practical alternative to the PaO2/FiO2 ratio in assessing oxygenation status. This validation suggests the potential for incorporating SpO2/FiO2 as a criterion for ARDS diagnosis, providing an accessible and widely available measurement option (16). Studies validate the utility of simple, readily available non-invasive markers such as SpO2 and FiO2 as good prognostic markers (17).
- Re-evaluation of bilateral versus unilateral opacities on chest imaging: The requirement for bilateral opacities on chest imaging as part of the Berlin definition has been subject to scrutiny. Evidence suggests that unilateral opacities may also be associated with significant respiratory impairment and could be included in a revised definition to capture a broader spectrum of ARDS cases (18).
- Use of ultrasound as an additional method for chest imaging: ultrasound has emerged as a valuable tool for assessing lung pathology and has shown promise in identifying lung consolidations and other findings associated with ARDS. Incorporating ultrasound as an additional imaging modality could enhance the diagnostic accuracy and utility of a revised ARDS definition.
- The lack of a pragmatic outcome measure for ARDS resolution contributes to prolonged therapeutic intervention resulting in a greater mortality rate (19).
The European Respiratory Society (ERS) clinical practice guidelines elaborate further on using HFNC as a first line of oxygen supplementation over conventional oxygen supplementation and non-invasive ventilation in hypoxemic respiratory failure and non-surgical patients with low risk of extubation failure. Amongst patients with a high risk of extubation failure or hypercapnic respiratory failure, a trial of non-invasive ventilation is recommended before HFNC use. In post-operative patients, HFNC use was non-inferior to conventional oxygen therapy amongst those with a low risk of pulmonary complications and non-inferior to non-invasive ventilation amongst patients with a high risk of pulmonary complications (20).
Epidemiology
Identifying the epidemiology of ARDS poses a significant challenge due to the need for a definitive confirmatory test, thereby relying on the established diagnostic criteria. This challenge is supported by the findings of a notable observational study conducted by Bellani et al., which reported that only 51% of ARDS cases were clinically recognized using the current diagnostic criteria (21). This underscores the difficulty in accurately identifying and capturing the true incidence of ARDS. Moreover, ARDS is intricately linked to the availability of Intensive care unit (ICU) beds and ventilatory support, which exhibit significant disparities across countries and regions. Consequently, reported incidences of ARDS exhibit a wide range of 3.7–81 per 100,000 person-years, accompanied by mortality rates spanning from 15% to 52% (18,22-25). The wide range of reported mortality rates also reflects the severity and heterogeneity of ARDS cases studied. Factors such as the underlying cause of ARDS, the severity of lung injury, comorbidities, and access to appropriate medical interventions can influence the outcome. Additionally, variations in treatment protocols and advancements in critical care practices over time can contribute to differences in reported mortality rates.
ARDS has been recognized as a clinical condition precipitated by various causes or risk factors. Non-pulmonary sepsis (infection in the bloodstream) and severe pneumonia are the most common contributors, accounting for approximately 40% of ARDS cases (3,5). Since the institution of conservative transfusion strategy, lung protective ventilation strategy, the incidence of ARDS secondary to trauma and blood product transfusion appears to be declining (3,5). On the other hand, new causes of ARDS have emerged in recent years. For example, e-cigarette use has been reported to induce significant lung injury and contribute to the development of ARDS (26,27). Additionally, throughout history, sporadic outbreaks or spikes of ARDS associated with specific viral infections have occurred. These include pandemics such as influenza, as well as outbreaks of severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and the most recent one caused by the novel coronavirus SARS-CoV-2, which led to the COVID-19 pandemic (28).
Unfortunately, ARDS mortality is high, with observational studies reporting 30% to 40% mortality (18,29). Development of ARDS associated with sepsis likely increases the risk of in-hospital mortality (30,31). Despite implementing a lung protective ventilation strategy, a multicenter retrospective analysis reported that barotrauma in COVID-19 infection was 13%, with higher in-hospital mortality rates (32,33).
Pathophysiology
ARDS has a complex pathophysiology that is yet to be clearly delineated. This is due to the limitations of the animal ARDS model and performing comprehensive mechanistic studies in humans proved to be ethically challenging due to the requirement of invasive studies and the potential risk involved. However, with decades of clinical research, the route of the ARDS-triggering injury can be crudely differentiated between direct lung injury and bloodstream-initiated pulmonary insult.
The alveolar-capillary barrier comprises the lung epithelial layer, the basement membrane, and the capillary endothelium. In its normal state, this structure is thin, facilitating efficient gaseous exchange between the alveoli in the lungs and the capillaries. However, in the context of ARDS, the alveolar-capillary barrier’s thickness increases due to injury. This thickening is recognized as a critical characteristic of ARDS. The changes in the barrier’s thickness can impede the gaseous exchange, leading to respiratory impairment.
The lung epithelium is composed of two types of cells: type 1 cells and type 2 cells. Type 1 cells are thin and possess a large surface area, enabling efficient gaseous exchange. On the other hand, type 2 cells secrete a complex mixture known as pulmonary surfactant (PS). PS plays a crucial role in maintaining normal respiratory mechanics by reducing alveolar surface tension to near-zero levels and preventing alveolar collapse. In cases of direct lung injury, damage to the epithelial layer can occur due to the adherence of molecules, triggering a proinflammatory response and potentially resulting in necrosis, contributing to alveolar flooding.
Furthermore, the impairment of type 2 cells due to this damage leads to a decline in surfactant production. Additionally, concomitant injury involving the shredding of the epithelial glycocalyx further exacerbates the proinflammatory response (34,35). The shredding of the lung epithelium has significant consequences, including the release of tissue factors such as endothelial protein C receptor and thrombomodulin. These tissue factors are crucial in the coagulation cascade and can lead to fibrin deposition within the affected area (36).
The permeability of capillaries is usually tightly regulated. However, in conditions such as sepsis or systemic inflammation, this regulation can be disrupted, leading to an increased gap between endothelial cells due to the upregulation of angiopoietin-2. Consequently, there is a net extravasation of fluid out of the vascular space and into the tissue. In addition to the changes in capillary permeability, circulating pathogens or proinflammatory cytokines can upregulate adherence proteins such as E-selectin and P-selectin. These proteins play a role in facilitating neutrophil adherence to the endothelial lining of blood vessels worsening the inflammatory process (37-39).
Subphenotypes
Although less clearly defined when compared to other pathologies, studies have demonstrated the presence of subphenotypes in ARDS. Calfee et al. describe the presence of two distinct sub-phenotypes in their latent class analysis of two randomized control trials, namely the ARMA trial and the ALVEOLI trial (40). These two phenotypes were characterized to have distinct natural histories, clinical and biological characteristics, and clinical outcomes. One of the most distinctive and clinically relevant features between the two phenotypes is the difference in their responses to treatment with higher PEEP vs. lower PEEP strategy. Phenotype 2 was identified to have a greater mortality risk when treated with a lower PEEP strategy compared to phenotype 1. Overall, phenotype two was characterized to have a more severe inflammatory state associated with metabolic acidosis, shock, and significantly poorer clinical outcomes (greater risk of organ failure, requirement of mechanical ventilation, and mortality). One of the interesting findings was the presence of elevated levels of plasma protein biomarkers such as interleukin 6, interleukin 8, tumor necrosis factor receptor 1, and plasminogen activator inhibitor-1 that could potentially serve as markers to identify the subphenotype and hence institute appropriate treatment strategies (40).
However, one of the disadvantages of using these clinical biomarkers is the limited availability of real-time assays for these biomarkers. Maddali et al. used a machine learning model to describe the two phenotypes using only readily available clinical variables as predictors (41). They also delineated a hyperinflammatory phenotype that responded well to a high PEEP strategy. Using these machine learning models in the future would help better risk stratify patients and institute appropriate management to improve outcomes.
PMD and PNX
Pathophysiology
The pressure gradient inside the normal pleural space is negative compared to atmospheric pressure. The surface tension between the parietal and visceral pleura allows the lung to expand outwards together with the chest wall and collapse due to elastic recoil. PNX and PMD are conditions characterized by air in abnormal locations within the chest cavity. It occurs when a full-thickness defect is present in the parietal and visceral pleura, and air is accumulated in the pleural space until there is no longer a pressure difference (Figure 1).
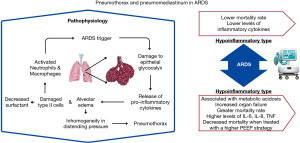
In normal lung parenchyma, the alveoli are intricately formed in an interdependent fashion to safeguard against overdistention during inspiration and collapse during expiration (42). However, in the case of ARDS, lung compliance is substantially reduced due to the accumulation of alveolar edema and an increase in fibrotic tissue resulting from inflammation. Given the heterogeneity of ARDS, the compliance of some lung regions may remain intact while others are severely affected (43,44). This disparity in compliance across the lung can be particularly hazardous, as decruited alveoli may lead to inhomogeneity in distending pressure during mechanical ventilation, placing greater stress on surrounding alveoli (45). Consequently, this heightened stress can contribute to the cleavage of cell-cell adhesion, increasing the risk of barotrauma.
Spontaneous PMD, also known as Hamman’s syndrome, was initially described by Macklin as “the movement of air along the sheaths of pulmonic blood vessels from the alveoli to the mediastinum”. This condition can be visualized using chest CT and often necessitates investigation into underlying causes due to its probable association with concomitant diseases (46). Secondary PMD in the setting of a trigger such as ARDS or procedure-related such as traumatic intubation occurs due to similar pathophysiology of alveolar rupture leading to air dissection along the bronchovascular sheaths and spreading into the mediastinum (47).
Notably, during the COVID-19 pandemic, we observed that some patients developed PNX/PMD that was not limited to positive airway pressure, suggesting that barotrauma is not solely the cause (48). Studies employing positron emission tomography/computed tomography (PET/CT) have shed light on the inflammatory aspect of ARDS. PET/CT scans with fluorodeoxyglucose (FDG) uptake has revealed increased metabolic activity in well-aerated lung regions, implying persistent inflammation despite lung-protective ventilation (21,49). This supported the observation during the COVID-19 pandemic, where the incidence of PNX/PMD was found to be sevenfold higher in CoV-ARDS patients than non-CoV-ARDS patients despite using the same ventilation strategy in both groups, as well as higher than expected incidence of barotrauma in COVID-19 patients without the need of invasive mechanical ventilation (50). Once alveoli are ruptured, air dissects along the connective tissue of the bronchovascular bundle and accumulates in the subpleural connective tissue forming a subpleural air cyst that leads to PNX and PMD if air dissects towards the hilum (51). Mediastinum emphysema was the early manifestation of barotrauma in 61% of ARDS patients, with 42% developing pneumothoraces within three days (52).
In addition to ventilation-related barotrauma, procedures often performed in ARDS patients, such as bronchoscopy and central line placement, can cause PNX if visceral pleura lacerations occur during the procedure. Some literature evidence suggests the development of PNX or PMD in ARDS patients, with some patients independently developing subpleural cysts (48,53). This could potentially explain the greater rate of spontaneous PMD observed in COVID-19 patients when compared to the general population (54).
Clinical features
Patients with ARDS typically exhibit sudden dyspnea and reduced arterial oxygen saturation within 72 hours. Signs such as tachypnea, tachycardia, and diffuse crackles are often observed on physical examination. If PNX or PMD occurs, distinctive features include asymmetrical lung expansion, decreased tactile fremitus, and a hyper resonant percussion note. Additional signs of hemodynamic instability and jugular venous distention may be evident in the case of tension PNX. In intubated patients, it is crucial to consider the possibility of PNX/PMD if there is an acute elevation of plateau and peak pressures beyond 30 cmH2O, ventilator asynchrony, or a sudden decrease in delivered tidal volume.
Diagnosis/imaging
PNX/PMD can be detected with chest X-ray by identifying a thin, white visceral pleural line without peripheral lung markings in erect films. However, in most intubated and critically ill patients, chest radiographs often can be obtained only in a supine or semi-recumbent position, making the identification of small PNX/PMD difficult but not impossible. In the supine position, the earliest and most subtle sign of PNX is often the presence of air in the anteromedial pleura (55), which may be seen as a subtle increase in density or lucency compared to the surrounding lung tissue.
CT scans may reveal PNX/PMD that are not readily apparent on plain chest film. With the incidence of PNX and PMD progressively increasing during the COVID pandemic, several tools emerged that served as possible indicators of impending barotrauma-related complications. Amongst them, one of the tools that was backed by literature was the Macklin effect. The Macklin effect is defined as the presence of air in the peribronchovascular space. It was intended to allow proper differentiation between respiratory and other causes of air leakage in the mediastinum that can be appreciated under CT scan and has been found to have accurately predicted the development of barotrauma with COVID-19 pneumonia. However, the result was yet to be validated in non-COVID-19 settings (56,57). It is essential to note that while CT scans can provide valuable information, the decision to transport critically ill patients to the CT scanner must be made cautiously and judiciously. Transportation of critically ill patients can be logistically challenging and may pose risks, especially for unstable patients who require close monitoring.
Point-of-care ultrasound (POCUS) has become increasingly favored for guiding the diagnosis of PNX/PMD due to its portability, real-time imaging capabilities, and ability to provide immediate information at the bedside. Here are some key sonographic findings with POCUS for PNX and PMD:
- PNX findings:
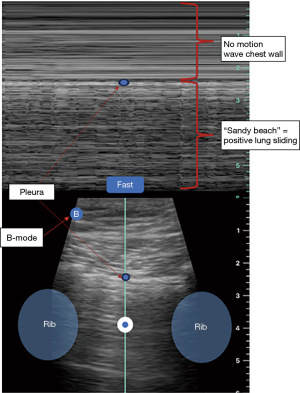
- Absence of lung sliding: normally, lung sliding is observed as a shimmering or gliding motion of the pleural line with respiration. This will be represented as a “sandy beach” appearance on M-mode (Figure 2) and will not be seen in patient with PNX.
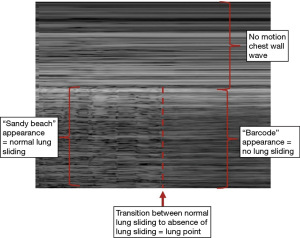
- Stratosphere sign: also known as the “barcode sign”, refers to the visualization of a continuous horizontal line (stratosphere) parallel to the pleural line, indicating the presence of air between the pleural layers. This sign can be seen in the absence of lung sliding due to the loss of contact between visceral and parietal pleura and is highly suggestive of PNX (Figure 3).
- Absence of B-lines: B-lines are comet-tail artifacts originating from the pleural line and extending to the ultrasound image’s bottom. They are commonly observed in lung ultrasound and indicate normal lung aeration. The absence of B-lines in a specific area can indicate air accumulation, as seen in PNX.
- “Lung point” (if present): the lung point is a specific finding seen in POCUS for PNX. It represents the point where the collapsed lung intermittently touches the chest wall during respiration. The presence of a lung point confirms the diagnosis of PNX (Figure 3).
- PMD findings:
- Visualized free air in the soft tissue of the neck: PMD can sometimes extend into the soft tissues of the neck, and on ultrasound, this appears as hyperechoic foci with ring-down artifacts, indicating the presence of air.
- Air gap sign: the air gap sign refers to the visualization of air within the mediastinum or pericardium, which can obscure normal cardiac structures.
Using POCUS to diagnose PNX/PMD can provide valuable information quickly, allowing for immediate clinical decision-making, especially in critical or emergency settings. It is important to note that POCUS is an operator-dependent modality. It was reported to have a high specificity, negative and positive predictive value while identifying PNX/PMD. However, the sensitivity of this modality was limited by the operator’s skill and increased the risk of misdiagnosis if misinterpretation occurs.
Management
There is limited literature that defines the management of PNX and PMD in ARDS patients (Table 4). The current treatment regimen ranges from simple clinical observations for small spontaneous pneumothoraces to thoracoscopic treatments for PNX with respiratory compromise (58). Conservative management is aimed at high-dose oxygen therapy, with studies demonstrating an increased resolution rate of PNX with high-dose oxygen therapy (59). Oxygen therapy is proposed to reduce the partial pressure of nitrogen in the alveolus compared with the pleural cavity. The difference in the pressure gradient for nitrogen accelerates the resolution (60). In patients with a large PNX or not responding to conservative measures, chest tube thoracostomy has proven useful (61). Amongst patients with a high rate of recurrences, chemical pleurodesis is the recommended treatment (62). It is usually achieved through chest tube administration or minimally invasive surgical techniques. Video-assisted thoracoscopic surgery (VATS) is often employed in applying pleurodesis since it enables the surgeon to completely visualize the pleural cavity and detect and treat pulmonary lesions before performing the pleurodesis (62). Ershadi et al. describe that 80.6% of pneumothoraces are managed with chest tubes, while 13.4% are managed with conservative measures and 6% with surgical intervention (63).
Table 4
| Management strategies |
| Supportive management (high dose oxygen therapy) |
| Chest tube thoracostomy |
| Video-assisted thoracoscopic surgery |
| Chemical pleurodesis |
| Prevention strategies |
| Use of neuromuscular blocking agents |
| Ventilator strategies |
| Lowered end-inspiratory pressures 35 cmH2O |
| Pulmonary compliance <30 mL/cmH2O |
| Advanced lung protective ventilation strategies |
| High frequency jet ventilation |
| High frequency oscillatory ventilation |
| Independent lung ventilation |
| Partial liquid ventilation |
| Extracorporeal membrane oxygenation |
PNX, pneumothorax; PMD, pneumomediastinum; ARDS, acute respiratory distress syndrome.
One of the significant complications encountered with treating pneumothoraces in ARDS is the formation of pulmonary air leaks. The literature demonstrates that pulmonary air leak complications increase mortality by 26% (64). Chest tube thoracostomy remains the first line for managing pulmonary air leaks. However, in the onset of persistent air leaks and other barotrauma-related complications such as bronchopleural fistula and bilateral PNX, surgical treatment becomes necessary. Surgery often involves an open thoracotomy, resection of the leaking parenchymal area with parietal pleurectomy, and application of sclerosing agents (58). The impact of ventilator management on the development of persistent air leaks has not been studied in detail. The retrospective study by Miyake et al. does not demonstrate any association between the development of persistent air leaks and ventilator pressures. One significant association inferred from the study was the greater risk of persistent air leak development amongst patients with high dynamic lung compliance and high tidal volume suggesting a pulmonary component contributing to the air leak complication rather than a ventilator component (65).
Prevention strategies
The absence of definitive curative strategies and the increased rate of mortality and morbidity associated with the development of PNX/PMD in ARDS patients emphasizes the significance of preventing complications. Several studies were undertaken to assess the impact of each risk factor on the incidence of PNX. All the ventilation parameters were studied, including peak inspiratory pressure, PEEP, respiratory rate, tidal volume, and minute ventilation. The end-inspiratory pressure [P(plat) or plateau pressure] is directly correlated with the incidence of PNX. There was a significant rate of PNX incidence when the end-inspiratory pressure exceeded 35 cmH2O, and pulmonary compliance dropped below 30 mL/cmH2O (66). These findings lead to the development of lung-protective strategies and the ARDSnet protocol to decrease the incidence of barotrauma-related complications. The efficacy of these treatment protocols has been backed by updated literature (67).
Other radiological evidence indicated PNX development, such as vesicular rarefactions, lucent lines or halos around vessels, pneumatoceles, and emphysematous changes (68).
One recent advance in preventing pneumothoraces in ARDS is the discovery of the utility of neuromuscular blocking agents. The study by Needham et al. demonstrated a decreased incidence of PNX in severe ARDS patients who received cisatracurium (69). Other studies also observed improved 90-day mortality, increased ventilator-free days, and ICU-free days when ARDS patients are treated with neuromuscular blocking agents (69-71).
Advanced lung protective ventilation strategies
With the advent of newer ventilation techniques, some have been hypothesized to help prevent barotrauma-related injury in severe ARDS. Their clinical application is yet to be assessed entirely, but several studies are investigating their utility.
High-frequency ventilation is one such mechanical ventilation model that has helped decrease the effects of pulmonary barotrauma. It is modeled by providing low maximal and high recruitment pressures using low tidal volumes delivered at constant mean airway pressures at high respiratory rates. The model aims to provide the beneficial effects of oxygenation and ventilation while eliminating the traumatic inflate-deflate cycle posed by conventional ventilation (72). The two most common forms of high-frequency ventilation are high-frequency oscillatory ventilation (HFOV) and high-frequency jet ventilation (HFJV). Studies have demonstrated that HFOV not only helps prevent the incidence of barotrauma and PNX but has also successfully managed pneumothoraces in ARDS patients (73,74). HFJV has also been reported to help manage PNX in ARDS (75).
Independent lung ventilation using a double-lumen tube allows one to independently aerate each lung with different ventilation parameters, thereby assisting in managing air leaks (76). Partial liquid ventilation using perfluorocarbon is also a suggested strategy. It has been shown to improve oxygenation and minimize lung damage safely (77). However, there are limited clinical studies that demonstrate the effectiveness in the treatment of PNX in ARDS patients.
Extracorporeal membrane oxygenation (ECMO) is a form of cardiopulmonary life-support, where the blood bypassed the heart and lungs and circulated outside of the body where gaseous exchange may take place and then reinfused back to the circulation. ECMO has been another significant factor that plays a role in the reduction of lung overdistension and consequent barotrauma-related complications. ECMO has been proven to effectively reduce the ventilation pressures such as peak and mean airway pressures, tidal lung volumes, and respiratory rate (78). There has been an increasing number of reports that ECMO significantly reduces the mortality and morbidity rate in ARDS patients (79,80). Although there tends to be a significant advancement in ventilation strategies to prevent barotrauma-related complications in ARDS, we lack studies that directly compare the utility of these methods.
Mortality
There is a significant mortality difference observed in ARDS patients who develop barotrauma-related complications compared to those who have not. Guven et al. describe a mortality rate of 40% in patients with barotrauma compared to the 29% observed in patients without barotrauma (81). These patients tend to have poorer prognoses secondary to prolonged hospitalization and an increased in-hospital and 90-day mortality. These findings were shown to be independent of age, sex, the severity of the disease as well as the need for respiratory support (32). One of the most critical findings noted in these studies was the increased development of PNX and PMD in patients with a greater time lapse between symptom onset and hospitalization. This prolonged length of respiratory disease could be contributing to more lung hyperinflammation. This is predominantly attributed to the increasingly difficult management of patients with persistent air leaks.
The relationship between the rate of mortality and the etiology of ARDS has been studied with great interest. One of the studies showed that ARDS contributes to 31.67% of hospital mortality, with the pulmonary cause of ARDS contributing to 44% and the extrapulmonary cause of ARDS contributing to 33.2% of the mortality (82). Among the four clinical phenotypes of ARDS, namely, the Sepsis and non-sepsis types and the pulmonary and extrapulmonary phenotypes, the mortality rate is highest in the sepsis-related pulmonary ARDS phenotype (83). In the early stage of ARDS, the pulmonary type is characterized by a worse impairment of gas exchange and a greater potential for lung recruitment when compared to the extrapulmonary type (84). This correlation between the high potential of lung recruitment and high mortality rates observed in pulmonary phenotype raises suspicion of an intrinsic mechanism that may contribute to the mortality rate. One of the direct explanations for this phenomenon is the potential for increased barotrauma observed as we tend to recruit more that in turn contributes to increased mortality.
ARDS in COVID-19 (Table 5)
Table 5
| Incidence of barotrauma is 15–40% |
| Pathophysiology: fibrin microthrombi → necrosis of pulmonary necrosis → cystic & cavitary lesion |
| Radiological findings |
| Unilateral infiltrative process |
| Diffuse bilateral ground glass opacities |
| Consolidative processes |
| Mortality rate of 40–50% |
| Not on any organ support, 8.2% |
| On invasive mechanical ventilation, 40.8% |
| On ECMO, 39% |
| On ventilatory support, vasoactive drugs, and renal replacement therapy, 71.6% |
COVID-19, coronavirus-19 disease; ARDS, acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation.
The incidence of barotrauma amongst ARDS patients is reported to be 6–7% (85). However, this rate significantly increases in the presence of a viral infection, especially in the coronavirus family. During the SARS epidemic, the rate of barotrauma increased to 12%; during the MERS epidemic, it soared as high as 30% (86). The COVID-19 pandemic created an unprecedented rate of 15–40% barotrauma risk in ARDS patients (87). One of the most important reasons for this increased rate of barotrauma associated with COVID-19 is the plethora of hyperinflammation associated with SARS-CoV-2 virus infection and the resultant diffuse alveolar injury. The diffuse alveolar injury is accompanied by fibrin microthrombi formation in the vascular area, leading to inflammation, consolidation, and necrosis in the pulmonary parenchyma. This subsequently leads to the formation of cavitary and cystic lesions in the lungs over time that can fistulate between the parenchyma and pleura, creating a spontaneous PNX. This was evidenced by Martinelli et al., who reported that 32% of patients with COVID-19 were reported to have PNX (88).
Alveolar rupture can occur in COVID-19 patients, which results in interstitial emphysema. This emphysema causes dissection along the bronchovascular sheath towards the mediastinum leading to PMD (89). Radiological findings that are usually associated with COVID-19 ARDS are widely variable, ranging from unilateral infiltrative process to diffuse bilateral ground glass opacities and consolidative processes (90).
Along with the increased incidence of PNX, the mortality rate associated with PNX in COVID-19 patients with ARDS is significantly high, with studies demonstrating an increased mortality rate of 40–50% (63,91). A complication of PNX or PMD in patients with COVID-19 and ARDS leads to greater length of hospitalization, higher mechanical ventilatory requirements, and greater in-hospital mortality (92). The mortality rate correlated with the severity of the disease, with patients not on any organ support having a mortality rate as low as 8.2% while patients on invasive mechanical ventilation had a mortality rate of 40.8%. Patients with multiorgan failure and requiring mechanical ventilatory support, vasoactive drugs, and renal replacement therapy had a significantly elevated mortality rate of 71.6%. ECMO utilization soared during the COVID-19 pandemic, showing a significant mortality benefit with a mortality rate of 39% (91). Deaths in the first year of the pandemic were enormously high due to the absence of consistent clinical guidelines in managing the COVID-19 illness (93). With a growing number of studies analyzing the prognostic influence of different risk factors, several drugs, such as aspirin use, were found to be associated with reduced mortality rate in COVID-19 patients (94).
Limitations
One of the significant limitations that we observed was the limited literature evidence studying the association between PNX, PMD, and ARDS patients. The advent of the COVID-19 pandemic led to several studies analyzing this association, but the generalizability of the results of the COVID-19 population to the general population needs to be more explicit. This review was limited to articles in the English language. Some of the recommendations are based on expert reviews and editorials that may be subject to bias.
Conclusions
This review provides an update on the barotrauma-related complications of PNX and PMD in patients with ARDS. These complications pose a significant mortality risk and worse prognosis due to the prolonged hospital stay. The frequent need for invasive ventilatory support creates a milieu of positive airway pressure-related complications, such as persistent air leaks that require extensive surgical management. High rates of pneumothoraces and PMD during the COVID-19 pandemic helped shed light on the obscure pathophysiology underlying the development of PNX and PMD in ARDS patients. Further research is needed for better risk stratification of ARDS patients at risk for developing PNX and PMD and hence institute appropriate prevention and treatment strategies.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://med.amegroups.com/article/view/10.21037/med-23-39/rc
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-23-39/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-23-39/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Doyle RL, Szaflarski N, Modin GW, et al. Identification of patients with acute lung injury. Predictors of mortality. Am J Respir Crit Care Med 1995;152:1818-24. [Crossref] [PubMed]
- Sloane PJ, Gee MH, Gottlieb JE, et al. A multicenter registry of patients with acute respiratory distress syndrome. Physiology and outcome. Am Rev Respir Dis 1992;146:419-26. [Crossref] [PubMed]
- Pepe PE, Potkin RT, Reus DH, et al. Clinical predictors of the adult respiratory distress syndrome. Am J Surg 1982;144:124-30. [Crossref] [PubMed]
- Fowler AA, Hamman RF, Good JT, et al. Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med 1983;98:593-7. [Crossref] [PubMed]
- Hudson LD, Milberg JA, Anardi D, et al. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med 1995;151:293-301. [Crossref] [PubMed]
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818-24. [Crossref] [PubMed]
- ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet 1967;2:319-23. [Crossref] [PubMed]
- Murray JF, Matthay MA, Luce JM, et al. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988;138:720-3. [Crossref] [PubMed]
- Riviello ED, Kiviri W, Twagirumugabe T, et al. Hospital Incidence and Outcomes of the Acute Respiratory Distress Syndrome Using the Kigali Modification of the Berlin Definition. Am J Respir Crit Care Med 2016;193:52-9. [Crossref] [PubMed]
- Matthay MA, Arabi Y, Arroliga AC, et al. A New Global Definition of Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2024;209:37-47. [Crossref] [PubMed]
- Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Estenssoro E, Dubin A, Laffaire E, et al. Impact of positive end-expiratory pressure on the definition of acute respiratory distress syndrome. Intensive Care Med 2003;29:1936-42. [Crossref] [PubMed]
- Esteban A, Fernández-Segoviano P, Frutos-Vivar F, et al. Comparison of clinical criteria for the acute respiratory distress syndrome with autopsy findings. Ann Intern Med 2004;141:440-5. [Crossref] [PubMed]
- Ferguson ND, Frutos-Vivar F, Esteban A, et al. Acute respiratory distress syndrome: underrecognition by clinicians and diagnostic accuracy of three clinical definitions. Crit Care Med 2005;33:2228-34. [Crossref] [PubMed]
- Rice TW, Wheeler AP, Bernard GR, et al. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest 2007;132:410-7. [Crossref] [PubMed]
- Chalmers SJ, Odeyemi YE, Lal A, et al. F(IO(2)) Trajectory as a Pragmatic Intermediate Marker in Acute Hypoxic Respiratory Failure. Respir Care 2021;66:1521-30. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Chalmers SJ, Lal A, Gajic O, et al. Timing of ARDS Resolution (TARU): A Pragmatic Clinical Assessment of ARDS Resolution in the ICU. Lung 2021;199:439-45. [Crossref] [PubMed]
- Oczkowski S, Ergan B, Bos L, et al. ERS clinical practice guidelines: high-flow nasal cannula in acute respiratory failure. Eur Respir J 2022;59:2101574. [Crossref] [PubMed]
- Bellani G, Messa C, Guerra L, et al. Lungs of patients with acute respiratory distress syndrome show diffuse inflammation in normally aerated regions: a [18F]-fluoro-2-deoxy-D-glucose PET/CT study. Crit Care Med 2009;37:2216-22. [Crossref] [PubMed]
- Sigurdsson MI, Sigvaldason K, Gunnarsson TS, et al. Acute respiratory distress syndrome: nationwide changes in incidence, treatment and mortality over 23 years. Acta Anaesthesiol Scand 2013;57:37-45. [Crossref] [PubMed]
- Li G, Malinchoc M, Cartin-Ceba R, et al. Eight-year trend of acute respiratory distress syndrome: a population-based study in Olmsted County, Minnesota. Am J Respir Crit Care Med 2011;183:59-66. [Crossref] [PubMed]
- Manzano F, Yuste E, Colmenero M, et al. Incidence of acute respiratory distress syndrome and its relation to age. J Crit Care 2005;20:274-80. [Crossref] [PubMed]
- Nolan S, Burgess K, Hopper L, et al. Acute respiratory distress syndrome in a community hospital ICU. Intensive Care Med 1997;23:530-8. [Crossref] [PubMed]
- Krishnasamy VP, Hallowell BD, Ko JY, et al. Update: Characteristics of a Nationwide Outbreak of E-cigarette, or Vaping, Product Use-Associated Lung Injury - United States, August 2019-January 2020. MMWR Morb Mortal Wkly Rep 2020;69:90-4. [Crossref] [PubMed]
- Layden JE, Ghinai I, Pray I, et al. Pulmonary Illness Related to E-Cigarette Use in Illinois and Wisconsin - Final Report. N Engl J Med 2020;382:903-16. [Crossref] [PubMed]
- Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239-42. [Crossref] [PubMed]
- Luhr OR, Antonsen K, Karlsson M, et al. Incidence and mortality after acute respiratory failure and acute respiratory distress syndrome in Sweden, Denmark, and Iceland. The ARF Study Group. Am J Respir Crit Care Med 1999;159:1849-61. [Crossref] [PubMed]
- Stapleton RD, Wang BM, Hudson LD, et al. Causes and timing of death in patients with ARDS. Chest 2005;128:525-32. [Crossref] [PubMed]
- Auriemma CL, Zhuo H, Delucchi K, et al. Acute respiratory distress syndrome-attributable mortality in critically ill patients with sepsis. Intensive Care Med 2020;46:1222-31. [Crossref] [PubMed]
- Bonato M, Fraccaro A, Landini N, et al. Pneumothorax and/or Pneumomediastinum Worsens the Prognosis of COVID-19 Patients with Severe Acute Respiratory Failure: A Multicenter Retrospective Case-Control Study in the North-East of Italy. J Clin Med 2021;10:4835. [Crossref] [PubMed]
- Chopra A, Al-Tarbsheh AH, Shah NJ, et al. Pneumothorax in critically ill patients with COVID-19 infection: Incidence, clinical characteristics and outcomes in a case control multicenter study. Respir Med 2021;184:106464. [Crossref] [PubMed]
- Rizzo AN, Haeger SM, Oshima K, et al. Alveolar epithelial glycocalyx degradation mediates surfactant dysfunction and contributes to acute respiratory distress syndrome. JCI Insight 2022;7:e154573. [Crossref] [PubMed]
- Zuo YY, Veldhuizen RA, Neumann AW, et al. Current perspectives in pulmonary surfactant--inhibition, enhancement and evaluation. Biochim Biophys Acta 2008;1778:1947-77. [Crossref] [PubMed]
- Wang L, Bastarache JA, Wickersham N, et al. Novel role of the human alveolar epithelium in regulating intra-alveolar coagulation. Am J Respir Cell Mol Biol 2007;36:497-503. [Crossref] [PubMed]
- Mulligan MS, Polley MJ, Bayer RJ, et al. Neutrophil-dependent acute lung injury. Requirement for P-selectin (GMP-140). J Clin Invest 1992;90:1600-7. [Crossref] [PubMed]
- Parikh SM, Mammoto T, Schultz A, et al. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med 2006;3:e46. [Crossref] [PubMed]
- Newman W, Beall LD, Carson CW, et al. Soluble E-selectin is found in supernatants of activated endothelial cells and is elevated in the serum of patients with septic shock. J Immunol 1993;150:644-54. [Crossref] [PubMed]
- Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2014;2:611-20. [Crossref] [PubMed]
- Maddali MV, Churpek M, Pham T, et al. Validation and utility of ARDS subphenotypes identified by machine-learning models using clinical data: an observational, multicohort, retrospective analysis. Lancet Respir Med 2022;10:367-77. [Crossref] [PubMed]
- Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 1970;28:596-608. [Crossref] [PubMed]
- Maunder RJ, Shuman WP, McHugh JW, et al. Preservation of normal lung regions in the adult respiratory distress syndrome. Analysis by computed tomography. JAMA 1986;255:2463-5. [Crossref] [PubMed]
- Pelosi P, D'Andrea L, Vitale G, et al. Vertical gradient of regional lung inflation in adult respiratory distress syndrome. Am J Respir Crit Care Med 1994;149:8-13. [Crossref] [PubMed]
- Cressoni M, Chiurazzi C, Gotti M, et al. Lung inhomogeneities and time course of ventilator-induced mechanical injuries. Anesthesiology 2015;123:618-27. [Crossref] [PubMed]
- Macklin CC. Transport of air along sheaths of pulmonic blood vessels from alveoli to mediastinum: clinical implications. Arch Intern Med 1939;64:913-26. [Crossref]
- Lal A, Mishra AK, Sahu KK, et al. Spontaneous Pneumomediastinum: Rare Complication of Tracheomalacia. Arch Bronconeumol (Engl Ed) 2020;56:185-6. [Crossref] [PubMed]
- Lal A, Mishra AK, Akhtar J, et al. Pneumothorax and pneumomediastinum in COVID-19 acute respiratory distress syndrome. Monaldi Arch Chest Dis 2021;91: [Crossref] [PubMed]
- Cressoni M, Chiumello D, Chiurazzi C, et al. Lung inhomogeneities, inflation and [18F]2-fluoro-2-deoxy-D-glucose uptake rate in acute respiratory distress syndrome. Eur Respir J 2016;47:233-42. [Crossref] [PubMed]
- Lemmers DHL, Abu Hilal M, Bnà C, et al. Pneumomediastinum and subcutaneous emphysema in COVID-19: barotrauma or lung frailty? ERJ Open Res 2020;6:00385-2020. [Crossref] [PubMed]
- Fleming WH, Bowen JC. Early complications of long-term respiratory support. J Thorac Cardiovasc Surg 1972;64:729-38. [Crossref] [PubMed]
- Gammon RB, Shin MS, Buchalter SE. Pulmonary barotrauma in mechanical ventilation. Patterns and risk factors. Chest 1992;102:568-72. [Crossref] [PubMed]
- Joynt GM, Antonio GE, Lam P, et al. Late-stage adult respiratory distress syndrome caused by severe acute respiratory syndrome: abnormal findings at thin-section CT. Radiology 2004;230:339-46. [Crossref] [PubMed]
- Melhorn J, Achaiah A, Conway FM, et al. Pneumomediastinum in COVID-19: a phenotype of severe COVID-19 pneumonitis? The results of the United Kingdom (POETIC) survey. Eur Respir J 2022;60:2102522. [Crossref] [PubMed]
- Rhea JT, vanSonnenberg E, McLoud TC. Basilar pneumothorax in the supine adult. Radiology 1979;133:593-5. [Crossref] [PubMed]
- Maccarrone V, Liou C, D'souza B, et al. The Macklin effect closely correlates with pneumomediastinum in acutely ill intubated patients with COVID-19 infection. Clin Imaging 2023;97:50-4. [Crossref] [PubMed]
- Paternoster G, Belmonte G, Scarano E, et al. Macklin effect on baseline chest CT scan accurately predicts barotrauma in COVID-19 patients. Respir Med 2022;197:106853. [Crossref] [PubMed]
- Terzi E, Zarogoulidis K, Kougioumtzi I, et al. Acute respiratory distress syndrome and pneumothorax. J Thorac Dis 2014;6:S435-42. [PubMed]
- Park CB, Moon MH, Jeon HW, et al. Does oxygen therapy increase the resolution rate of primary spontaneous pneumothorax? J Thorac Dis 2017;9:5239-43. [Crossref] [PubMed]
- Henderson Y, Henderson MC. The absorption of gas from any closed space within the body: particularly in the production of atelectasis and after pneumothorax. Arch Intern Med 1932;49:88-93. [Crossref]
- Ulutas H, Celik MR, Gulcek I, et al. Management of spontaneous pneumothorax in patients with COVID-19. Interact Cardiovasc Thorac Surg 2022;34:1002-10. [Crossref] [PubMed]
- Nistor CE, Pantile D, Stanciu-Gavan C, et al. Diagnostic and Therapeutic Characteristics in Patients with Pneumotorax Associated with COVID-19 versus Non-COVID-19 Pneumotorax. Medicina (Kaunas) 2022;58:1242. [Crossref] [PubMed]
- Ershadi R, Rafieian S, Salehi M, et al. COVID-19 and spontaneous pneumothorax: a survival analysis. J Cardiothorac Surg 2023;18:211. [Crossref] [PubMed]
- Martínez-Escobar S, Ruiz-Bailén M, Lorente-Acosta MJ, et al. Pleurodesis using autologous blood: a new concept in the management of persistent air leak in acute respiratory distress syndrome. J Crit Care 2006;21:209-16. [Crossref] [PubMed]
- Miyake N, Igarashi Y, Nakae R, et al. Ventilator management and risk of air leak syndrome in patients with SARS-CoV-2 pneumonia: a single-center, retrospective, observational study. BMC Pulm Med 2023;23:251. [Crossref] [PubMed]
- Finfer S, Rocker G. Alveolar overdistension is an important mechanism of persistent lung damage following severe protracted ARDS. Anaesth Intensive Care 1996;24:569-73. [Crossref] [PubMed]
- Miller MP, Sagy M. Pressure characteristics of mechanical ventilation and incidence of pneumothorax before and after the implementation of protective lung strategies in the management of pediatric patients with severe ARDS. Chest 2008;134:969-73. [Crossref] [PubMed]
- Johnson TH, Altman AR, McCaffree RD. Radiologic considerations in the adult respiratory distress syndrome treated with positive end expiratory pressure (PEEP). Clin Chest Med 1982;3:89-100. [Crossref] [PubMed]
- Needham CJ, Brindley PG. Best evidence in critical care medicine: The role of neuromuscular blocking drugs in early severe acute respiratory distress syndrome. Can J Anaesth 2012;59:105-8. [Crossref] [PubMed]
- Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363:1107-16. [Crossref] [PubMed]
- Lagneau F. Indications and uses of neuromuscular blocking agents in the ICU. Ann Fr Anesth Reanim 2008;27:567-73. [Crossref] [PubMed]
- Meyers M, Rodrigues N, Ari A. High-frequency oscillatory ventilation: A narrative review. Can J Respir Ther 2019;55:40-6. [Crossref] [PubMed]
- Galvin I, Krishnamoorthy R, Saad RS. Management of advanced ARDS complicated by bilateral pneumothoraces with high-frequency oscillatory ventilation in an adult. Br J Anaesth 2004;93:454-6. [Crossref] [PubMed]
- Martinón Torres F, Rodríguez Núñez A, Jaimovich DG, et al. High-frequency oscillatory ventilation in pediatric patients. protocol and preliminary results. An Esp Pediatr 2000;53:305-13. [PubMed]
- Smith DW, Frankel LR, Derish MT, et al. High-frequency jet ventilation in children with the adult respiratory distress syndrome complicated by pulmonary barotrauma. Pediatr Pulmonol 1993;15:279-86. [Crossref] [PubMed]
- Sawulski S, Nestorowicz A, Wośko J, et al. Independent lung ventilation for treatment of post-traumatic ARDS. Anaesthesiol Intensive Ther 2012;44:84-8. [PubMed]
- Anzueto A, Melo J. Acute respiratory distress syndrome. Liquid ventilation. Respir Care Clin N Am 1998;4:679-94. [PubMed]
- Manert W, Haller M, Briegel J, et al. Venovenous extracorporeal membrane oxygenation (ECMO) with a heparin-lock bypass system. An effective addition in the treatment of acute respiratory failure (ARDS). Anaesthesist 1996;45:437-48. [PubMed]
- Banach M, Soukup J, Bucher M, et al. High frequency oscillation, extracorporeal membrane oxygenation and pumpless arteriovenous lung assist in the management of severe ARDS. Anestezjol Intens Ter 2010;42:201-5. [PubMed]
- Mauri T, Foti G, Zanella A, et al. Long-term extracorporeal membrane oxygenation with minimal ventilatory support: a new paradigm for severe ARDS? Minerva Anestesiol 2012;78:385-9. [PubMed]
- Guven BB, Erturk T, Kompe Ö, et al. Serious complications in COVID-19 ARDS cases: pneumothorax, pneumomediastinum, subcutaneous emphysema and haemothorax. Epidemiol Infect 2021;149:e137. [Crossref] [PubMed]
- Agarwal R, Aggarwal AN, Gupta D, et al. Etiology and outcomes of pulmonary and extrapulmonary acute lung injury/ARDS in a respiratory ICU in North India. Chest 2006;130:724-9. [Crossref] [PubMed]
- Wang Y, Zhang L, Xi X, et al. The Association Between Etiologies and Mortality in Acute Respiratory Distress Syndrome: A Multicenter Observational Cohort Study. Front Med (Lausanne) 2021;8:739596. [Crossref] [PubMed]
- Coppola S, Froio S, Marino A, et al. Respiratory Mechanics, Lung Recruitability, and Gas Exchange in Pulmonary and Extrapulmonary Acute Respiratory Distress Syndrome. Crit Care Med 2019;47:792-9. [Crossref] [PubMed]
- Anzueto A, Frutos-Vivar F, Esteban A, et al. Incidence, risk factors and outcome of barotrauma in mechanically ventilated patients. Intensive Care Med 2004;30:612-9. [Crossref] [PubMed]
- Kao HK, Wang JH, Sung CS, et al. Pneumothorax and mortality in the mechanically ventilated SARS patients: a prospective clinical study. Crit Care 2005;9:R440-5. [Crossref] [PubMed]
- McGuinness G, Zhan C, Rosenberg N, et al. Increased Incidence of Barotrauma in Patients with COVID-19 on Invasive Mechanical Ventilation. Radiology 2020;297:E252-62. [Crossref] [PubMed]
- Martinelli AW, Ingle T, Newman J, et al. COVID-19 and pneumothorax: a multicentre retrospective case series. Eur Respir J 2020;56:2002697. [Crossref] [PubMed]
- Liu K, Zeng Y, Xie P, et al. COVID-19 with cystic features on computed tomography: A case report. Medicine (Baltimore) 2020;99:e20175. [Crossref] [PubMed]
- Lal A, Mishra AK, Sahu KK. CT chest findings in coronavirus disease-19 (COVID-19). J Formos Med Assoc 2020;119:1000-1. [Crossref] [PubMed]
- Domecq JP, Lal A, Sheldrick CR, et al. Outcomes of Patients With Coronavirus Disease 2019 Receiving Organ Support Therapies: The International Viral Infection and Respiratory Illness Universal Study Registry. Crit Care Med 2021;49:437-48. [Crossref] [PubMed]
- Devarajan A, Tekin A, Sakata KK, et al. Pneumomediastinum and pneumothorax in COVID-19 pneumonia: a matched case-control study. Chest 2022;162:A1383-4. [Crossref]
- Johnson SW, Garcia MA, Sisson EKQ, et al. Hospital Variation in Management and Outcomes of Acute Respiratory Distress Syndrome Due to COVID-19. Crit Care Explor 2022;10:e0638. [Crossref] [PubMed]
- Lal A, Garces JPD, Bansal V, et al. Pre-hospital Aspirin Use and Patient Outcomes in COVID-19: Results from the International Viral Infection and Respiratory Illness Universal Study (VIRUS). Arch Bronconeumol 2022;58:746-53. [Crossref] [PubMed]
Cite this article as: Teo YX, Geetha HS, Mishra AK, Lal A. Pneumomediastinum and pneumothorax in acute respiratory distress syndrome (ARDS) patients: a narrative review. Mediastinum 2024;8:3.