
Superior vena cava obstruction and cardiovascular implantable electronic devices—a new era of leadless devices
Introduction
Cardiovascular implantable electronic devices (CIEDs), such as pacemakers for bradycardia and implantable cardioverter defibrillators (ICDs) for tachycardia, are usually implanted by inserting a lead from the subclavian vein into the cardiac cavity via the superior vena cava (SVC) and connecting it to a generator that is implanted above (or below or in other locations) the pectoralis major muscle. However, transvenous lead placement may be difficult if the SVC is obstructed by a tumor or for some other reason. Moreover, SVC syndrome may occur after the lead is inserted even if the SVC was intact before the implantation. This article outlines these problems.
Device therapy for patients with SVC obstruction
SVC syndrome is a syndrome in which severe stenosis or obstruction of the SVC causes impaired venous blood return from the upper body, resulting in congestion and edema of the head, face, neck, and upper body. The most common presenting symptoms include facial and neck edema, distended neck and chest veins, watering eyes, and dizziness, particularly when leaning forward (1). The clinical presentation varies depending on the severity, location, and rapidity of the onset of the obstruction and the establishment of collateral veins. The diagnosis of SVC syndrome is based on the clinical symptoms and imaging. Imaging modalities include chest radiography, contrast-enhanced computed tomography (CT), duplex ultrasound, conventional catheter-based digital subtraction venography, and magnetic resonance venography (1). If a patient with SVC obstruction requires CIED therapy, two strategies should be considered: (I) treatment of the SVC obstruction, and (II) placement of a CIED in which the lead line does not pass through the SVC.
Avoidance of SVC obstruction
The maximum number of leads that can be implanted in a vein with an acceptably low risk of complications is a controversial topic (2). There are few data on the lead burden that results in venous access issues and SVC syndrome, and consensus documents are based on expert opinion as to the numbers of abandoned leads that justify extraction; i.e., a total of more than four leads on one side or five leads through the SVC (3). Performing a venography examination prior to the puncture is important in cases of SVC occlusion or pre-existing venous thrombosis.
Treatment of SVC obstruction
Malignancy accounts for about 70% of the cases of SVC syndrome. The most common benign causes are cardiac device therapy and catheterization, such as in patients on dialysis (1). The strategy of treatment for patients with SVC obstruction requiring device therapy is illustrated in Figure 1. Treatment of SVC occlusion is based on treatment of the underlying disease causing the occlusion and the mechanism of occlusion (thrombotic or non-thrombotic). If treatment of the underlying disease is difficult or the need for device therapy is urgent, a CIED that does not require a transvenous lead should be considered.
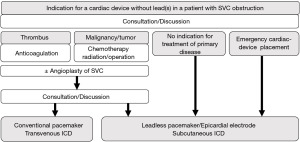
Symptomatic therapies for SVC occlusion include thrombolytic therapy in cases with a thrombotic cause of occlusion and balloon dilation, stenting, and surgical SVC angioplasty in the non-thrombotic cases. Pothineni et al. described four cases of angioplasty and implanted transvenous lead placement in patients with SVC syndrome (4). Although stenting for SVC syndrome had a favorable outcome in a meta-analysis (5), there is insufficient prognostic data for transvenous lead placement after angioplasty. Discussions should be held again after the SVC has been unobstructed, and the indications for and methods of CIED should be thoroughly discussed.
For patients with tumors, chemotherapy, radiation therapy, and surgery should be considered as appropriate; if there is no indication for treatment of the underlying disease causing the SVC obstruction or if urgent cardiac device therapy is needed, use of a CIED that does not require a transvenous lead will be necessary.
CIED for SVC obstruction
CIEDs can be divided into two categories: (I) pacemakers for bradycardia and (II) ICDs for defibrillation of tachycardia. Devices that do not require a transvenous lead have been developed (6). Cardiac resynchronization therapy (CRT) is also included in CIEDs with transvenous leads, but the potential issues that are associated with CRT are discussed below.
Pacemakers for bradycardia
Pacemakers that do not require a transvenous lead include open chest surgery with myocardial electrodes and leadless pacemakers.
Myocardial electrodes do not pass through the intravascular space. Ito et al. reported open chest biopsy and implantation of a myocardial electrode in a patient with primary cardiac lymphoma who had SVC syndrome and sinus failure (7). Maseda Uriza et al. (8) reported a case of SVC syndrome with a pacemaker lead in which the lead was removed, stents were placed, and a myocardial lead was implanted.
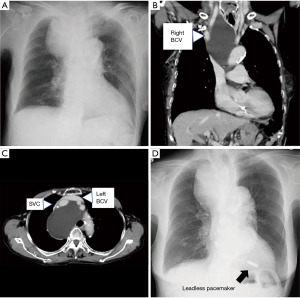
Leadless pacemakers have become widely used in recent years (9). Hirano et al. reported a case of complete atrioventricular block complicated with SVC syndrome due to malignant lymphoma (10). In this case, a leadless pacemaker was inserted during chemotherapy, and the atrioventricular block improved 74 days after chemotherapy. We experienced a case of complete atrioventricular block associated with a mediastinal tumor (11). The patient was very old and there was no indication for treatment of the tumor; thus, a leadless pacemaker was implanted (Figure 2).
ICDs for tachyarrhythmias
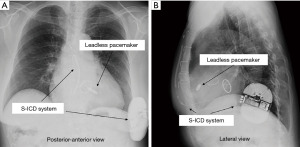
ICDs are implanted for the tachyarrhythmias (ventricular tachycardia and ventricular fibrillation); ICDs can terminate tachyarrhythmias by delivering electric shock(s) between the generator and a shock lead implanted in the right ventricle. Subcutaneous implantable cardioverter defibrillator (S-ICD), in which the defibrillation lead is implanted subcutaneously rather than intravascularly, have also been developed (12), and have been shown to be as safe and effective as conventional ICDs (13). S-ICDs have the following two disadvantages: (I) antitachycardia pacing cannot be performed with an S-ICD, and (II) unlike ICDs with transvenous leads, S-ICDs do not have a pacemaker function for bradycardia except after shock. Ito et al. reported a case in which an S-ICD was implanted in a patient with ventricular tachycardia who had a history of mechanical valve infective endocarditis and a leadless pacemaker was placed for subsequent bradycardia (Figure 3) (14). In addition, attempts are being made to develop a novel modular cardiac rhythm management system consisting of a communicating antitachycardia pacing-enabled leadless pacemaker and an S-ICD (15). In the future, therefore, it should be possible to combine an S-ICD with a leadless pacemaker that has the same functionality as an ICD with transvenous leads. Problems with inappropriate therapy of the S-ICD have been reported (16), and attempts are being made to reduce the problems with these devices (17).
Management of terminally ill patients and others
In terminally ill patients, in justified cases, external endocavitary stimulation may be used. Indications for pacemaker or ICD implantation are included in the American College of Cardiology/American Heart Association guidelines for patients with indications for permanent pacing but also for those with significant comorbidities such that pacing therapy is unlikely to provide meaningful clinical benefit; if a patient’s care goals strongly preclude pacemaker therapy, the implantation or replacement of a pacemaker should not be performed (18). In patients who are expected to have a shortened life span because of a terminal progressive illness, the benefits of pacing support may not be realized and are unlikely to positively impact the overall outcome (19). For patients with advanced cancer, in addition to a subcutaneous ICD, the possibility of implanting a defibrillating electrode into the azygos vein or pericardial sac retrosternally as well as a defibrillating vest might be considered an option.
Therapeutic strategy for SVC obstruction after lead implantation
The therapeutic strategy for SVC obstruction after lead implantation is depicted in Figure 4.
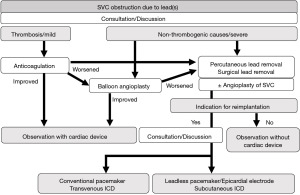
Pacemakers and ICDs account for 7.5% of all cases of thrombotic SVC syndrome, and the number of cases has been increasing over time (20). Thrombosis, mechanical stress, infection, and inflammation are possible mechanisms of lead-induced SVC occlusion (21). Riley et al. summarized information on the treatment of lead-related SVC syndrome (22). Medical treatment for SVC syndrome often results in recurrence (5–33%), while surgical treatment results in relatively few recurrences (11%), but the invasiveness of the procedure is problematic.
Anticoagulation
A high D-dimer level may indicate that thrombosis is a contributing factor in the mechanism of SVC occlusion, and anticoagulation may be indicated.
Mumoli et al. treated a patient with SVC syndrome 2 years after pacemaker implantation with a week of full dose subcutaneous enoxaparin and subsequent anticoagulation with edoxaban 60 mg, and after 3 months, the patient was free of symptoms and a chest CT angiography revealed complete resolution of the thrombosis, and the anticoagulant was interrupted (23). The thrombosis was not acute, and Mumoli et al. hypothesized that anticoagulation can restore a favorable balance between thrombosis persistence and physiologic fibrinolysis, leading to thrombus resolution (23). Fukui et al. treated a patient with SVC syndrome 2 years after pacemaker implantation and administered rivaroxaban; they observed no recurrence of thrombosis over 1 year of rivaroxaban therapy (24).
Balloon angioplasty, stenting
If symptoms are severe, catheterization is preferred for symptom improvement; Eberhardt et al. performed vasodilation for SVC syndrome in a patient more than 10 years after pacemaker implantation and reported no problems after 1 year of treatment with rivaroxaban 20 mg (25). Pham also reported a case of balloon dilation for SVC obstruction and insertion of a new lead in a patient with SVC occlusion after 5 years (26). Stenting without lead removal has also been performed, but can potentially cause failure of the lead (27).
Surgical angioplasty
Surgical angioplasty is invasive but has the advantage of reliably treating SVC obstruction, such as when a wire cannot pass through the obstruction. Hodges et al. reported a case of SVC syndrome 3 years after pacemaker implantation in which a leadless pacemaker was implanted under direct vision after surgical SVC repair (28).
Percutaneous lead removal
In recent years, percutaneous lead removal has evolved and is recommended in guidelines (2). Arora et al. performed lead removal in 13 of 17 patients with SVC syndrome. All patients underwent revascularization (of the remaining four, three patients were treated with venoplasty alone, and 1 patient underwent surgical SVC reconstruction), and all were confirmed to have no recurrence at 1 year (29). Gabriels et al. summarized results over a longer follow-up (median of 5.5 years) in 16 patients with SVC syndrome who underwent transvenous lead extractions (30). In addition to transvenous lead extraction and percutaneous treatment, 13 patients (81.3%) were managed with long-term anticoagulation therapy, but 4 patients (25%) had recurrent symptoms between 5 months and 2 years after extraction. These patients were managed either conservatively or with further balloon angioplasty and stenting. One patient required an emergent repair with a surgical reconstruction due to an SVC tear that occurred during the extraction and placement of an epicardial lead. Manual lead removal may cause venous or cardiac perforation, and it is safer to remove the lead using a device such as a laser sheath. Anticoagulation, venoplasty, and surgical interventions alone have been abandoned due to the high risk of recurrences, and thus transvenous lead extraction followed by venoplasty with or without stenting are a reasonable first-choice approach for SVC syndrome (27).
In a retrospective analysis of 3,002 venograms from patients awaiting transvenous lead extraction, Czajkowski et al. observed that SVC occlusion was rare (31). Their research group also described risk factors for lead-related venous obstruction and the influence of lead-related venous obstruction on the complexity and outcomes of lead extraction (32,33). Clinicians need to be familiar with these risk factors and review patients’ venography findings to ensure safe lead removal.
Perspectives
Further data must be accumulated to clarify the long-term prognosis of device implantation after treatment of SVC occlusion. In addition, transvenous lead extraction is now widely used for device-related SVC obstruction, and this procedure also merits further accumulation of data. Ventricular single chamber pacing (VVI), single lead atrial synchronous ventricular pacing mode (VDD) leadless pacemakers are currently available, but dual chamber pacing (DDD) is also being developed (34). CRT for patients with heart failure that does not require a left ventricular lead is also being studied (35), but currently this strategy requires a right ventricular lead. The development of CRT without leads is eagerly anticipated.
Conclusions
In patients with SVC syndrome, treatment of the SVC occlusion should be based on the individual pathophysiology, and depending on the indications and urgency of the case, treatment with CIEDs that do not require transvenous leads should be considered.
Acknowledgments
The author thanks Dr. Hiroaki Watanabe and Dr. Masashi Kamioka (Jichi Medical University School of Medicine) for their clinical contributions.
Funding: None.
Footnote
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-23-33/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-23-33/coif). T.K. reports research and education support from Medtronic, Japan Lifeline, and Abbott. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Azizi AH, Shafi I, Shah N, et al. Superior Vena Cava Syndrome. JACC Cardiovasc Interv 2020;13:2896-910. [Crossref] [PubMed]
- Kusumoto FM, Schoenfeld MH, Wilkoff BL, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017;14:e503-51. [Crossref] [PubMed]
- Bongiorni MG, Burri H, Deharo JC, et al. 2018 EHRA expert consensus statement on lead extraction: recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: endorsed by APHRS/HRS/LAHRS. Europace 2018;20:1217. [Crossref] [PubMed]
- Pothineni NVK, Chahal CAA, Frankel DS, et al. Percutaneous recanalization of superior vena cava occlusions for cardiac implantable electronic device implantation: Tools and techniques. Heart Rhythm 2020;17:2010-5. [Crossref] [PubMed]
- Aung EY, Khan M, Williams N, et al. Endovascular Stenting in Superior Vena Cava Syndrome: A Systematic Review and Meta-analysis. Cardiovasc Intervent Radiol 2022;45:1236-54. [Crossref] [PubMed]
- Khan K, Kim JA, Gurgu A, et al. Innovations in Cardiac Implantable Electronic Devices. Cardiovasc Drugs Ther 2022;36:763-75. [Crossref] [PubMed]
- Ito K, Nishimura Y, Tanaka H, et al. Epicardial pacemaker implantation for sick sinus syndrome in a patient with supra vena cava obstructed by a primary cardiac lymphoma. J Cardiol Cases 2020;21:234-7. [Crossref] [PubMed]
- Maseda Uriza R, Jurado-Román A, Jimenez Díaz J, et al. Hybrid Approach for the Treatment of Superior Vena Cava Syndrome Induced by Pacemaker. Ann Thorac Surg 2017;104:e131-2. [Crossref] [PubMed]
- Reynolds D, Duray GZ, Omar R, et al. A Leadless Intracardiac Transcatheter Pacing System. N Engl J Med 2016;374:533-41. [Crossref] [PubMed]
- Hirano F, Okamura A, Kato M, et al. Successful Chemotherapy With Early Leadless Pacemaker Implantation for a Giant Malignant Lymphoma Complicated by Unstable Atrioventricular Block and Superior Vena Cava Syndrome. Circ J 2022;86:1480. [Crossref] [PubMed]
- Kabutoya T, Imai Y, Aoyama Y, et al. Leadless Pacemaker Implantation for a Super-elderly Woman with a Mediastinal Tumor. Intern Med 2022;61:1545-7. [Crossref] [PubMed]
- Weiss R, Knight BP, Gold MR, et al. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation 2013;128:944-53. [Crossref] [PubMed]
- Burke MC, Gold MR, Knight BP, et al. Safety and Efficacy of the Totally Subcutaneous Implantable Defibrillator: 2-Year Results From a Pooled Analysis of the IDE Study and EFFORTLESS Registry. J Am Coll Cardiol 2015;65:1605-15. [Crossref] [PubMed]
- Ito R, Kondo Y, Winter J, et al. Combination of a leadless pacemaker and subcutaneous implantable cardioverter defibrillator therapy for a Japanese patient with prosthetic valve endocarditis. J Arrhythm 2019;35:311-3. [Crossref] [PubMed]
- Tjong FVY, Koop BE. The modular cardiac rhythm management system: the EMPOWER leadless pacemaker and the EMBLEM subcutaneous ICD. Herzschrittmacherther Elektrophysiol 2018;29:355-61. [Crossref] [PubMed]
- Lambiase PD, Barr C, Theuns DA, et al. Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the EFFORTLESS S-ICD Registry. Eur Heart J 2014;35:1657-65. [Crossref] [PubMed]
- Brisben AJ, Burke MC, Knight BP, et al. A new algorithm to reduce inappropriate therapy in the S-ICD system. J Cardiovasc Electrophysiol 2015;26:417-23. [Crossref] [PubMed]
- Kusumoto FM, Schoenfeld MH, Barrett C, et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, and the Heart Rhythm Society. Circulation 2019;140:e333-81. [Crossref] [PubMed]
- Kusumoto FM, Schoenfeld MH, Barrett C, et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2019;140:e382-e482. Erratum in: Circulation 2019;140:e506-8. [Crossref] [PubMed]
- Mir T, Uddin M, Shafi O, et al. Thrombotic superior vena cava syndrome: a national emergency department database study. J Thromb Thrombolysis 2022;53:372-9. [Crossref] [PubMed]
- Malyshev Y, Ayzenberg S, Sahni S, et al. Narrow Escape: A Novel Approach to the Endovascular Treatment of Superior Vena Cava Syndrome Secondary to Pacemaker Leads with Excellent Long-term Outcomes. Cureus 2020;12:e7249. [Crossref] [PubMed]
- Riley RF, Petersen SE, Ferguson JD, et al. Managing superior vena cava syndrome as a complication of pacemaker implantation: a pooled analysis of clinical practice. Pacing Clin Electrophysiol 2010;33:420-5. [Crossref] [PubMed]
- Mumoli N, Mazzone A, Evangelista I, et al. Superior vena cava syndrome after pacemaker implantation treated with direct oral anticoagulation. Thromb J 2021;19:84. [Crossref] [PubMed]
- Fukui T, Ogasawara N. Oral anticoagulation therapy for pacemaker lead-induced superior vena cava syndrome. Eur Heart J Case Rep 2022;6:ytac433. [Crossref] [PubMed]
- Eberhardt F, Bunck AC, Codjambopoulo P, et al. Benign vena cava superior syndrome in patients with cardiac implantable electronic devices: Presentation and management. HeartRhythm Case Rep 2020;6:549-53. [Crossref] [PubMed]
- Pham LT. Treatment of pacemaker-induced superior vena cava syndrome by venoplasty with a coronary balloon. J Arrhythm 2021;37:1351-3. [Crossref] [PubMed]
- Domenichini G, Le Bloa M, Carroz P, et al. New Insights in Central Venous Disorders. The Role of Transvenous Lead Extractions. Front Cardiovasc Med 2022;9:783576. [Crossref] [PubMed]
- Hodges K, Tuohy S, Miletic K, et al. Superior vena cava reconstruction and implantation of a leadless pacemaker for management of pacemaker-induced superior vena cava syndrome. HeartRhythm Case Rep 2019;5:539-41. [Crossref] [PubMed]
- Arora Y, Carrillo RG. Lead-related superior vena cava syndrome: Management and outcomes. Heart Rhythm 2021;18:207-14. [Crossref] [PubMed]
- Gabriels J, Chang D, Maytin M, et al. Percutaneous management of superior vena cava syndrome in patients with cardiovascular implantable electronic devices. Heart Rhythm 2021;18:392-8. [Crossref] [PubMed]
- Czajkowski M, Jacheć W, Polewczyk A, et al. Severity and Extent of Lead-Related Venous Obstruction in More Than 3000 Patients Undergoing Transvenous Lead Extraction. Vasc Health Risk Manag 2022;18:629-42. [Crossref] [PubMed]
- Czajkowski M, Jacheć W, Polewczyk A, et al. The Influence of Lead-Related Venous Obstruction on the Complexity and Outcomes of Transvenous Lead Extraction. Int J Environ Res Public Health 2021;18:9634. [Crossref] [PubMed]
- Czajkowski M, Jacheć W, Polewczyk A, et al. Risk Factors for Lead-Related Venous Obstruction: A Study of 2909 Candidates for Lead Extraction. J Clin Med 2021;10:5158. [Crossref] [PubMed]
- Knops RE, Reddy VY, Ip JE, et al. A Dual-Chamber Leadless Pacemaker. N Engl J Med 2023;388:2360-70. [Crossref] [PubMed]
- Okabe T, Hummel JD, Bank AJ, et al. Leadless left ventricular stimulation with WiSE-CRT System - Initial experience and results from phase I of SOLVE-CRT Study (nonrandomized, roll-in phase). Heart Rhythm 2022;19:22-9. [Crossref] [PubMed]
Cite this article as: Kabutoya T. Superior vena cava obstruction and cardiovascular implantable electronic devices—a new era of leadless devices. Mediastinum 2024;8:1.


