
Thymic en-bloc resection with veins: case demonstrations and review of the literature
Introduction
The management of invasive thymic neoplasms has evolved in the last few decades. A major determinant of long-term outcomes in these cases is the completeness of resection (1). The involvement of the major vessels of the mediastinum, in particular the superior vena cava (SVC), was previously a contraindication to resection due to the poor long-term prognosis and lack of suitable replacements. That paradigm has shifted with the advent of adjuvant treatments, new conduit materials, and a growing experience of resection of these vessels en bloc with thoracic malignancies (2-4). The Masaoka-Koga classification is used to stage thymic malignancies. Select patients with stage III (macroscopic invasion into neighboring organs such as pericardium and great vessels) and stage IV disease (pleural, pericardial or lymph node metastasis) should be considered for thymectomy with en-bloc resection of the involved venous structures (5-7).
Case 1
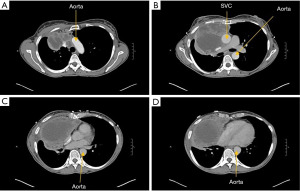
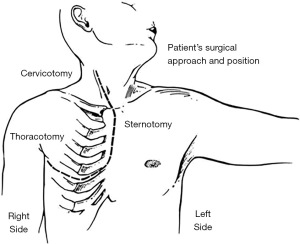
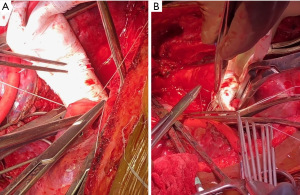
Patient was a 53-year-old woman with a history of tobacco use disorder and no other significant medical history who presented with a cough, intermittent fevers, and unintentional weight loss and was found on computed tomography (CT) chest to have a 10.5 cm × 10.5 cm × 15.8 cm heterogenous anterior mediastinal mass that was compressing the right upper lobe bronchus. The mass extended from the diaphragm to the base of the neck. Mediastinal lymphadenopathy, pleural and pericardial effusions, and multiple hepatic lesions were also noted. She underwent a biopsy via Chamberlain procedure and was diagnosed with thymoma. Given the extent of her disease, the patient underwent four cycles of neoadjuvant chemotherapy with cyclophosphamide, adriamycin, and cisplatin. She had a poor response with restaging CT scan demonstrating a minimally decreased tumor size with no change in mass effect (Figure 1). The patient then underwent a right thoraco-sternotomy (hemi-clamshell) through the 6th intercostal space (Figure 2). The mass was encasing the SVC and the left innominate vein, and an en-bloc resection was performed of the mass, right lung parenchyma, SVC, left innominate vein, proximal right innominate vein, and azygous vein. The SVC and the two innominate veins were repaired with 14-mm Gore-Tex bifurcating limbs that were sewn to a 20-mm graft. The azygous was sewn to the posterior aspect of the 20-mm graft (Figure 3).
Case 2
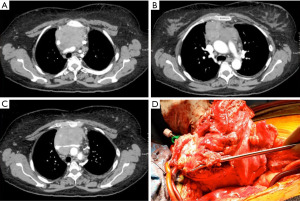
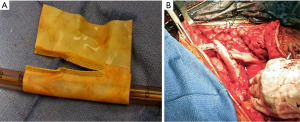
A 50-year-old woman presented with chest pain and on workup was found to have a large anterior mediastinal mass encasing the great veins (Figure 4). At the time of surgery, upon palpation of the lower table of the sternum, it was clear the tumor was invading the chest wall. She underwent en-bloc resection of the chest wall with the brachiocephalic veins and SVC. During the dissection, a shunt was placed from the right internal jugular vein to the right atrium. The reconstruction was performed with tailored bovine pericardium. We initially placed a conduit from the right internal jugular to the proximal SVC. Then, the remainder of the vessels were reconstructed and anastomosed to the SVC replacement (Figure 5).
Preoperative workup
An appropriate preoperative workup is essential in the management of patients with invasive thymic malignancies undergoing potential mediastinal venous resection and reconstruction. Appropriate pre-operative pulmonary and cardiac testing should be done. The most common imaging modality included in the workup is a contrast CT scan. This can give an idea of the extent of tumor involvement and aid in determining a surgical plan. Usually with regards to the surgical anatomy, a contrast CT scan is sufficient, and other tests such as a magnetic resonance imaging (MRI) or angiography are not necessary but can be helpful (3). In some cases, a biopsy may also be indicated, particularly when differentiating the histology of thymoma from thymic carcinoma.
For thymic neoplasms requiring resection of the SVC or innominate veins, determining the stage of the disease is crucial. This typically includes a positron emission tomography-CT (PET-CT) scan. Because phrenic nerve involvement typically requires resection of the nerve, bilateral phrenic nerve involvement would require careful pulmonary assessment prior to surgery and plans for bilateral diaphragm plication. Given the complexity of these cases, a multidisciplinary approach is often needed to individualize the treatment plan and determine the appropriate neoadjuvant or adjuvant therapy (2).
Operative approach
The operative approach used should be individualized, should maximize exposure of the critical structures, and should be familiar to the surgeon. The vast majority of these cases are done via a sternotomy, although a hemi-clamshell or a cervico-sternotomy are reasonable options (2,3,5-7).
Prior to selecting the optimal operative approach, it’s important to understand the limitations. For example, when a thoraco-sternotomy is used, there is limited access to the contralateral chest. Therefore, if the tumor is large and bulky, the inability to get access to both sides of the mediastinum may be an important limiting factor to attaining an R0 resection.
Others have reported minimally invasive approaches utilizing video-assisted thoracoscopy, although long-term follow-up has been limited in these reports (9-11). For the two aforementioned cases, a minimally invasive approach would not be feasible given the bulky nature of the tumors.
Lower extremity large bore intravenous access is necessary, and we frequently use a femoral vein central line as this provides reliable intravenous access throughout the case. A double lumen endotracheal tube may also be required for single lung ventilation.
After the initial dissection of the tumor, involvement of the SVC and surrounding structures should be assessed. This can be done by mobilizing the tumor and delivering it away from the mediastinum. The thoracotomy extension of the hemi-clamshell allows for lateral exposure to the heart and great vessels to aid in determination of resectability of the tumor. The SVC, the azygous vein, and the innominate veins should be dissected and encircled with vessel loops when possible. It may be necessary to get venous control distally. Additionally, the phrenic nerve should be mobilized and preserved if possible; however, it is often involved and is therefore sacrificed. There is some debate regarding the maximum involvement of the SVC that will permit a partial resection. Some surgeons advocate for no more than 30% involvement, stating increased risk of kinking, thrombosis and occlusion (5-7), while other surgeons advocate for less than 50% (2-4). When performing a partial resection, a partial occlusion clamp can be utilized and the SVC can be resected en bloc with the tumor. The SVC can then be repaired either primarily if a small resection was performed or with autologous pericardium, autologous vein, or prosthesis if a larger resection was done (2,11,12).
Complete SVC resection and reconstruction
If greater than 30–50% of the circumference of the SVC is involved, a complete resection and reconstruction is necessary. This requires clamping of the SVC, which should ideally be done above the azygous vein to preserve flow. We typically administer 5,000 units of heparin intravenously prior to clamping. Although the azygous vein can be ligated (2,3), we typically re-implant the azygous as the increase in flow through the conduit may aid long-term patency.
Clamping of the SVC may result in hemodynamic instability, which can be managed with pressors and intravenous fluid. It may also lead to cerebral edema and thrombosis, which can partly be alleviated with the reverse Trendelenburg position. It is therefore critical to maintain close communication with anesthesia colleagues to closely monitor for any changes in intracranial pressure. More frequently, however, flow through the SVC is chronically impaired due to the tumor, and thus clamping may be well tolerated. Tolerance can be determined using cerebral near infrared spectroscopy (i.e., cerebral oximetry), a non-invasive device that measures the absorption of infrared light through the tissue to determine venous oxygen saturation. This serves as a marker for brain oxygenation. Additionally, we monitor for development of plethora, which is an indication that clamping is not tolerated. The ideal clamp time is unclear, but we try to limit the time to less than 30 minutes. Some authors have safely clamped for 44 minutes, whereas animal models have safely tolerated times up to 60 minutes (13,14).
Since we cannot predict the time needed to successfully perform the resection and reconstruction, we routinely opt for a venous shunt for larger tumors. Options for venous shunting include a caval shunt, internal jugular vein to femoral vein shunt, and a shunt between the right innominate vein and the right atrium (15). In our experience, one must frequently go distally on the veins for the location of the inflow for the shunt. Our preference is to put the outflow into the right atrium, although the femoral vein can be used when that is not accessible. More advanced support such as cardiopulmonary bypass and extracorporeal membrane oxygenation (ECMO) are typically not necessary for most cases but can be helpful especially when the tumor is invading the atrium or aorta.
The involvement of the SVC will also determine the anatomy of the reconstruction. In cases where the innominate veins are not involved, a graft can be placed from the proximal SVC to the distal SVC. If the innominate confluence is involved, a number of options are available. A graft can be placed between the left innominate vein and the right atrium to establish venous drainage after which the SVC and innominate confluence can then be resected (2). A benefit of this technique is the early establishment of superior venous drainage while the SVC is resected, but the graft is more likely to kink given the steeper angle to the right atrium (3). Alternatively, we ligate the left innominate vein and place the graft from the right innominate vein to the SVC or the right atrium. Some authors routinely conduct bilateral reconstruction (7,14), citing improved drainage with decreased risk of brain edema. This is also our preferred method. Ligation of one of the innominate veins may lead to upper extremity swelling and development of neurologic symptoms although these are often temporary and managed conservatively as collateral flow from the azygous, hemiazygous and internal mammary veins develop (16).
A variety of conduit materials have been described to replace the SVC and the innominate veins: spiral saphenous vein, tubularized autologous pericardium, bovine or porcine pericardium, polytetrafluoroethylene (PTFE), and even aortic homografts (2,3,5,12,14). The optimal conduit is debated. Jiang and colleagues recently reported on their series of six patients who had SVC replacement with autologous pericardium. Five patients survived, two of whom developed severe SVC syndrome. None of the patients developed graft thrombosis in a 55-month average follow-up period (17).
In 1991, Dartevelle and his colleagues published their results of 22 patients who had SVC involvement from lung cancer or a mediastinal tumor and subsequently underwent a concomitant resection and reconstruction of the SVC with a PTFE graft. Everyone received anticoagulation therapy for at least 6 months. One patient died postoperatively and another developed mediastinitis that was treated with an omentopexy. The overall actuarial survival rate was 48% at 5 years with a 60% survival rate in those with mediastinal tumors. One graft occlusion occurred in the postoperative period and another 14 months later (4).
A more recent study by Oizumi and coauthors describes the outcomes of 12 patients who also had a combined resection and reconstruction of the SVC with a PTFE graft. Four patients had SVC syndrome preoperatively. Bilateral reconstruction was performed for nine patients, a single right-sided bypass for one patient, and a Y-shaped bypass for another. Anticoagulants were not administered postoperatively. There were no postoperative deaths. Of the 22 grafts, 3 (14%) became occluded (14).
Our current preference is to use autologous vein for simple reconstructions to minimize the risk of thrombosis. An autologous saphenous vein can be fashioned into a patch or a luminal conduit; however, this can be time consuming and the availability of the saphenous vein is a limitation. It is best to have another surgeon harvest the vein and fashion the conduit while the operative resection is being performed. In general, for more complex scenarios requiring significant reconstruction, we use ringed PTFE grafts because it is easy to manipulate, resists external compression, and is associated with a low incidence of complications. From the preoperative contrast imaging, we can estimate the size of the graft needed. Despite this, we typically size the grafts intraoperatively for more accurate measurements.
After vascular clamps are placed for proximal and distal control, and resection of the SVC and possibly the right innominate vein is done, we first perform the proximal anastomosis with a running 5-0 prolene suture. The distal anastomosis is then performed also with a 5-0 prolene suture. Prior to tying down the suture, the graft is de-aired by opening the proximal clamp. Once the anastomosis is complete, the distal clamp is then released.
Postoperative management, complications, and long-term outcomes
Depending on the extent of the venous resection, our patients are either transferred to our stepdown unit or to our intensive care unit (ICU) to monitor for major immediate postoperative complications. Antibiotics are continued for 24 hours. If a prosthetic SVC replacement is placed, we routinely administer anticoagulation for at least 3 months. Because there is always a concern for thrombosis when a non-endothelialized graft is used, we continue some form of low dose anticoagulation or antiplatelet medication, such as aspirin, indefinitely.
Complete R0 resection ranges from 80% to 100% (5,7,18). The postoperative morbidity rate has been reported between 31% and 52% (5-7) and a mortality rate around 8%, although these studies have been more focused on thymic resection with SVC resection (5,6). For patients undergoing thymectomy with mediastinal venous resection, the complications have included pulmonary infection, hemothorax, chylothorax, atelectasis, acute respiratory distress syndrome, and need for blood transfusions (5-7). Respiratory failure is also a common complication (2,12), and aggressive pulmonary toileting is therefore crucial. Graft infection can also occur but is typically secondary to an infectious pulmonary process (2). Reports on long-term 5-year survival range from 44–59% (5-7).
Many factors contribute to complications and long-term survival. There is little doubt that an R0 resection is associated with a better long-term survival than an incomplete resection (6,18). Lung invasion has also been linked with poor outcomes (18). While induction treatment is less often indicated and should be a multidisciplinary decision, we favor its use as one cannot predict the response to treatment. Other areas are more controversial, such as the need for a complete SVC replacement. Some have shown that complete SVC replacement with a prosthetic is associated with an increased risk of death (19) while others have reported that it is equally as safe as partial SVC resection and repair (12). We opt to conduct a partial resection when feasible but acknowledge the utility of a complete resection when necessary.
Conclusions
The management of thymic neoplasms involving the SVC and innominate veins has changed drastically in the last few decades. Where these were once strong contraindications to surgical resection, extirpative surgery is now a reasonable option in select patients who are fit to undergo major cardiothoracic surgery and who have Masaoka stage IIIB and IV thymic neoplasms. Given the improvements in adjuvant therapies and conduit materials, the safety and durability of SVC resection and repair or reconstruction should be considered in a multidisciplinary approach.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Editorial Office, Mediastinum for the series “Venous Surgery of the Mediastinum”.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-20-69/coif). The series “Venous Surgery of the Mediastinum” was commissioned by the editorial office without any funding or sponsorship. A.S. served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this article and accompanying images. The written consents are available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wilkins KB, Sheikh E, Green R, et al. Clinical and pathologic predictors of survival in patients with thymoma. Ann Surg 1999;230:562-72; discussion 572-4. [Crossref] [PubMed]
- Garcia A, Flores RM. Surgical management of tumors invading the superior vena cava. Ann Thorac Surg 2008;85:2144-6. [Crossref] [PubMed]
- Okereke IC, Kesler KA. Superior vena cava and innominate vein reconstruction in thoracic malignancies: single-vein reconstruction. Semin Thorac Cardiovasc Surg 2011;23:323-5. [Crossref] [PubMed]
- Dartevelle PG, Chapelier AR, Pastorino U, et al. Long-term follow-up after prosthetic replacement of the superior vena cava combined with resection of mediastinal-pulmonary malignant tumors. J Thorac Cardiovasc Surg 1991;102:259-65. [Crossref] [PubMed]
- Maurizi G, Poggi C, D'Andrilli A, et al. Superior Vena Cava Replacement for Thymic Malignancies. Ann Thorac Surg 2019;107:386-92. [Crossref] [PubMed]
- Sun Y, Gu C, Shi J, et al. Reconstruction of mediastinal vessels for invasive thymoma: a retrospective analysis of 25 cases. J Thorac Dis 2017;9:725-33. [Crossref] [PubMed]
- Zhang Z, Huang M, Pan X. Prosthetic Reconstruction of Superior Vena Cava System for Thymic Tumor: A Retrospective Analysis of 22 Cases. Thorac Cardiovasc Surg 2021;69:165-72. [Crossref] [PubMed]
- Mugabure B, Eizaguirre M, Gonzalez S, et al. Thoracic Epidural Morphine for Postoperative Analgesia after Hemiclamshell Incision in Castleman Disease. Open J Anesthesiol 2013;3:156-60. [Crossref]
- Yano M, Okuda K, Kawano O, et al. Thoracoscopic Thymectomy with Tangential Partial Resection of the Innominate Vein. Ann Thorac Cardiovasc Surg 2017;23:207-10. [Crossref] [PubMed]
- Xu N, Gu Z, Ji C, et al. Thoracoscopic thymectomy with partial superior vena cava resection for locally advanced thymomas. J Thorac Dis 2019;11:438-44. [Crossref] [PubMed]
- Xu X, Qiu Y, Pan H, et al. Resection of the sidewall of superior vena cava using video-assisted thoracic surgery mechanical suture technique. J Thorac Dis 2016;8:612-6. [Crossref] [PubMed]
- Leo F, Bellini R, Conti B, et al. Superior vena cava resection in thoracic malignancies: does prosthetic replacement pose a higher risk? Eur J Cardiothorac Surg 2010;37:764-9. [Crossref] [PubMed]
- Masuda H, Ogata T, Kikuchi K. Physiological changes during temporary occlusion of the superior vena cava in cynomolgus monkeys. Ann Thorac Surg 1989;47:890-6. [Crossref] [PubMed]
- Oizumi H, Suzuki K, Banno T, et al. Patency of grafts after total resection and reconstruction of the superior vena cava for thoracic malignancy. Surg Today 2016;46:1421-6. [Crossref] [PubMed]
- Dai W, Dong J, Zhang H, et al. Superior vena cava replacement combined with venovenous shunt for lung cancer and thymoma: a case series. J Thorac Dis 2018;10:363-70. [Crossref] [PubMed]
- McPhee A, Shaikhrezai K, Berg G. Is it safe to divide and ligate the left innominate vein in complex cardiothoracic surgeries? Interact Cardiovasc Thorac Surg 2013;17:560-3. [Crossref] [PubMed]
- Jiang S, Hu H, Guo C, et al. Thoracic tumor resection combined with SVC replacement using autologous pericardium. World J Surg Oncol 2019;17:227. [Crossref] [PubMed]
- Tang EK, Chang JM, Chang CC, et al. Prognostic Factor of Completely Resected and Pathologic T3 N0 M0 Thymic Epithelial Tumor. Ann Thorac Surg 2021;111:1164-73. [Crossref] [PubMed]
- Spaggiari L, Magdeleinat P, Kondo H, et al. Results of superior vena cava resection for lung cancer. Analysis of prognostic factors. Lung Cancer 2004;44:339-46. [Crossref] [PubMed]
Cite this article as: Young JS, DeBarros M, Singh A, Marshall MB. Thymic en-bloc resection with veins: case demonstrations and review of the literature. Mediastinum 2024;8:5.


