Postoperative radiotherapy for thymic epithelial tumors: a narrative review
Introduction
Background
Thymic epithelial tumors (TETs) are rare primary tumors originating from the anterior mediastinum and include thymomas and thymic carcinomas. The incidence of thymomas is 1.5 cases per million people, and that of thymic carcinoma is 0.3 cases per million people (1,2). Thymomas have a good prognosis with a 5-year overall survival (OS) rate of 90%, whereas that of thymic carcinomas is 55%, and a higher stage is associated with worse OS (3-6).
The Masaoka-Koga staging system has been commonly used for management determination and prognosis estimation of TETs (7,8). It focuses on the local invasive extent of the primary tumor (stages I–III), and pleural, pericardial, lymphogenous, or hematogenous metastases are all included in stage IV, as TETs spread locally and rarely develop lymphatic dissemination. Surgical resection is the main treatment strategy for stages I–III and IV TETs.
Pathological findings are closely associated with the prognosis of TET and are heterogeneous. According to the fifth edition of the World Health Organization (WHO) classification of the thymus and mediastinum, TETs are categorized as type A thymoma, type AB thymoma, thymoma type B1-B2-B3, several other minor thymoma subtypes, and carcinomas, including squamous cell carcinoma and adenocarcinoma (9); 10-year OS for each subtype were 100, 100, 85, 85, 65, and 40% for type A, AB, B1, B2, and B3 thymomas, and thymic carcinomas, respectively (10,11). Geographic differences exist in the frequency of WHO histological subtypes, which impact recurrence (12).
When patients with TETs undergo surgical resection, postoperative radiotherapy (PORT) is often administered to improve local control, depending on the pathological findings of the stage and the residual tumor (13).
Rationale and knowledge gap
Owing to the rarity of TETs, no randomized controlled trials (RCTs) have been conducted to confirm the efficacy of PORT in TETs, and the current evidence levels are not high and are mainly based on retrospective studies. According to the National Comprehensive Cancer Network guidelines, completely resected Masaoka-Koga stage I thymomas do not require adjuvant therapy, whereas PORT is administered for TETs with microscopic (R1) or macroscopic (R2) residual tumors (13). For completely resected stage II–IV TETs, the guidelines recommend discussion by a multidisciplinary tumor board (MTB) to determine the patient’s treatment strategy because the efficacy of PORT in this area remains controversial.
The Réseau Tumeurs Thymiques et Cancer (RYTHMIC), a nationwide network for TETs in France, has prospectively gathered data to determine whether decisions on PORT made at the MTBs align with RYTHMIC guidelines and whether they are ultimately implemented in patient care (14). Among 241 patients with stage I–III disease, the MTB’s decision regarding PORT was not made in accordance with the ESMO/RYTHMIC guidelines in 20 patients. When the MTB recommended PORT in cases where the guidelines would have advised against it, a clear explanation for the inconsistency with the guidelines was not found; however, the cases were stage II thymomas with WHO type B2 or stage IIA thymomas with WHO type AB thymomas. Thus, the efficacy of PORT in TETs for these subjects could be considered a gray zone in the guidelines, where different MTBs would make different decisions.
Objective
This narrative review aimed to evaluate and summarize the current literature regarding PORT for TETs in terms of indications for PORT, radiotherapy techniques, and toxicities. This review sheds light on this understudied area by providing information on the current ongoing trials and recommendations for future research. We present this article in accordance with the Narrative Review reporting checklist (available at https://med.amegroups.com/article/view/10.21037/med-23-38/rc).
Methods
A summary of the search strategies is provided in Table 1. N.K. developed and executed the PubMed search on August 5th, 2023. The reproducible search strategies for creating a narrative review are presented in Table 2. The studies included in the review met the following eligibility criteria: (I) articles focusing on TETs; (II) articles that included topics on PORT; and (III) articles with English abstracts available online. The exclusion criteria were as follows: (I) case reports or review articles; (II) articles written in languages other than English; and (III) articles on thymic neuroendocrine tumors.
Table 1
| Items | Specification |
|---|---|
| Date of search (specified to date, month and year) | August 5th, 2023 |
| Databases and other sources searched | PubMed |
| Search terms used | radiotherapy, adjuvant, postoperative, thymic, thymoma |
| Timeframe | Since August 1st, 1992, until August 5th, 2023 |
| Inclusion and exclusion criteria | Inclusion criteria |
| (I) Articles on thymic epithelial tumors excluding thymic neuroendocrine tumor | |
| (II) Articles on postoperative radiotherapy | |
| Exclusion criteria | |
| (I) Articles written in non-English language | |
| (II) Case reports, review articles, or guidelines | |
| (III) Articles published >30 years ago | |
| (IV) Pre-clinical studies | |
| Selection process | N.K. conducted the selection independently and Y.M. reviewed the process |
Table 2
| Search | Query | Items |
|---|---|---|
| #1 | Has abstract | 24,918,516 |
| “hasabstract” (All Fields) | ||
| #2 | Adjuvant radiotherapy OR Postoperative radiotherapy | 425,765 |
| (“radiotherapy, adjuvant”(MeSH Terms) OR (“radiotherapy”(All Fields) AND “adjuvant”(All Fields)) OR “adjuvant radiotherapy”(All Fields) OR (“adjuvant”(All Fields) AND “radiotherapy”(All Fields))) OR ((“postoperative period”(MeSH Terms) OR (“postoperative”(All Fields) AND “period”(All Fields)) OR “postoperative period”(All Fields) OR “postop”(All Fields) OR “postoperative”(All Fields) OR “postoperatively”(All Fields) OR “postoperatives”(All Fields)) AND (“radiotherapy”(MeSH Terms) OR “radiotherapy”(All Fields) OR “radiotherapies”(All Fields) OR “radiotherapy”(MeSH Subheading) OR “radiotherapy s”(All Fields))) | ||
| #3 | Thymic (ti) OR Thymoma (ti) | 16,097 |
| “Thymic”(Title) OR “Thymoma”(Title) | ||
| #4 | #1 AND #2 AND #3 | 488 |
Indications for PORT
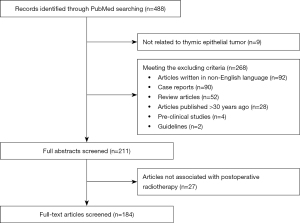
One hundred eighty-four articles were identified in the search (Figure 1). As no RCTs have assessed the efficacy of PORT, established rationales are based on retrospective studies of large databases. Although multi- or single-institutional retrospective studies have smaller sample sizes than large database studies, they can offer more detailed results. We review both types of studies and highlight their strengths.
Resected TET
Three pathological indications for PORT in resectable TETs have been discussed in the literature: pathological staging, WHO classification of histological subtypes, and resectional status (margin status).
Masaoka or Masaoka-Koga staging
The Masaoka or Masaoka-Koga staging system has been widely used in pathological staging to determine the indications for PORT in TETs. In contrast, the International Thymic Malignancy Interest Group (ITMIG), European Society of Thoracic Surgeons, Japanese Association of Research in Thymus, and Chinese Alliance for Research on Thymomas developed an international database, which resulted in the development of the TNM stage classification. It provides information on lymphatic involvement and tumor dissemination in addition to tumor invasion extent, and is comparable or superior in grouping TETs for predicting prognosis and guiding clinical management (15-17). However, previous reports on PORT were largely based on Masaoka or Masaoka-Koga staging, and a paradigm shift is occurring from the traditional Masaoka-Koga system to a TNM system. In this section, we outline the benefits of PORT according to the Masaoka or Masaoka-Koga stages. In Table 3, we present previous database studies that assessed the efficacy of PORT on OS, which have yielded conflicting results.
Table 3
| Authors | N | Primary | Stage | Database | Masaoka or Masaoka-Koga staging | 5-year OS in PORT vs. no PORT in stage II–IV | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | IIA | IIB | III | IV | ||||||
| Fernandes et al. (18) | 1,334 | Car | I–IV | SEER | NS (I–IIA) | NS | S (III–IV) | Median, 134 vs. 115 months (IIB) | ||
| Patel et al. (19) | 1,464 | Thy | I–IV | SEER | NS | S (II–III) | NA | 64% vs. 53% (II–III) | ||
| Weksler et al. (20) | 476 | Thy | III | SEER | – | – | – | NS | – | Median, 127 vs.105 months |
| Mariano et al. (21) | 171 | Thy | I–IV | BCCAR | NA | NS (II) | NA | NA | NA | |
| Ruffini et al. (22) | 2,265 | Car | I–IV | ESTS | S | 69% vs. 61% | ||||
| Omasa et al. (23) | 1,265 | TET | II–III | JART | – | NS (II) | NS | – | 97% vs. 96% (II, Thy) 93% vs. 90% (III, Thy) 91% vs. 87% (II, Car) 65% vs. 64% (III, Car) |
|
| Hishida et al. (24) | 306 | Car | I–IV | JART | NS (I–IV) | 78% vs. 74% | ||||
| Lim et al. (25) | 529 | Thy | IIB–IV | SEER | – | – | NS | S | S | NA |
| Fu et al. (26) | 329 | Car | I–IV | ChART | NS (R0, I–II) | S (R0) | S (R0) | NA | ||
| S (R1–2, I–IV) | ||||||||||
| Wang et al. (27) | 1,850 | Thy | I–IV | ChART | NS (I–IV) | NA | ||||
| Liu et al. (28) | 1,546 | TET | I–III | ChART | NS | NS (II) | NS | – | 90% vs. 96% | |
| Rimner et al. (29) | 1,263 | Thy | II–III | ITMIG | – | S (R0) | S (R0) | – | 97% vs. 93% (II) 92% vs. 76% (III) |
|
| Jackson et al. (30) | 4,056 | TET | I–IV | NCDB | NS (I–IIA) | S (Thy), NS (Car) | S (Thy), NS (Car) | NA | NA | |
| Lim et al. (31) | 312 | Car | I–IV | SEER | NS (I–II) | S | NS | NA | ||
| Mou et al. (32) | 2,234 | Thy | I–IV | SEER | NS (I–IIA) | NS | S (III–IV) | 75.4% vs. 62.9% (III–IV) | ||
| Bian et al. (33) | 1,272 | Thy | I–IV | SEER | NS | S (IIA–III) | S | NA | ||
| Gu et al. (34) | 1,087 | TET | I–II* | ChART | NS (I–II) | – | – | NA | ||
| Kim et al. (35) | 632 | Car | IIB–III | NCDB | – | – | S NS (R0) |
S | – | NA |
| Mou et al. (36) | 2,236 | Thy | I–IV | SEER | NS (I–IIA) | NS | S (III–IV) | NA | ||
| Wu et al. (37) | 216 | Car | I–IV | SEER | NS (I–IV) | NA | ||||
| Muslim et al. (38) | 1,120 | Thy | IIB–IV | SEER | – | – | NS† | S† | NS† | NA |
| Lococo et al. (39) | 203 | Car | I–IV | ESTS | S | 74% vs. 55% | ||||
| Zhang et al. (40) | 2,558 | TET | I–IV | SEER | NS (I–IIA) | S | S (Thy), NS (Car) | 82% vs. 75% (IIB, Thy) 66% vs. 46% (III–IV, Thy) 72% vs. 61% (IIB, Car) 38% vs. 23% (III–IV, Car) |
||
| Lin et al. (41) | 700 | Thy | IIB–III | SEER | – | – | NS | S | – | NA |
*, UICC stage I (equal to Masaoka stages I and II); †, disease-specific survival, not OS. OS, overall survival; PORT, postoperative radiotherapy; Car, thymic carcinoma; SEER, Surveillance, Epidemiology, and End Results; NS, not significant; S, significant; Thy, thymoma; BCCAR, British Columbia Cancer Agency Registry; NA, not available; ESTS, European Society of Thoracic Surgeons; TET, thymic epithelial tumor; JART, Japanese Association for Research on the Thymus; ChART, Chinese Alliance for Research of Thymoma database; ITMIG, International Thymic Malignancies Interest Group ; NCDB, National Cancer Database.
One of the largest retrospective database analyses using the National Cancer Data Base (NCDB) investigated 4,056 patients who underwent surgery for stage I–IV TETs (30). PORT positively correlated with improved OS in patients diagnosed with either thymoma or thymic carcinoma. For patients with stage IIB or III thymoma, PORT significantly increased OS. Among the subsets with margin-negative stage IIB thymoma, PORT was still associated with better OS. In patients with thymic carcinoma, PORT was significantly correlated with increased OS across the entire cohort. When classified into stages I–IIA, IIB, and III, no significant differences were noted, although a slight increase in OS was observed in patients with stage III disease. In another study using the ITMIG database, Rimner et al. reported an OS benefit with PORT in patients with completely resected stage II and III thymoma (29).
In contrast, a Surveillance, Epidemiology, and End Results (SEER) database study, which focused on 1,334 patients with thymoma between 1973 and 2005, did not show an OS benefit of PORT in patients with stage IIB disease (18). Lim et al. used a more recent patient cohort from the same database and reported that PORT in stages III–IV was associated with improved OS; however, no corresponding efficacy was observed in stage IIB (25). The Japanese Association for Research on the Thymus Database Study, which included 1,265 patients with TETs, showed that PORT improved recurrence-free survival (RFS) for stage II–III thymic carcinoma, but did not improve OS and RFS for stage II–III thymoma (23).
Five meta-analyses reported PORT for TETs: two TETs, two thymomas, and one thymic carcinoma. Two meta-analyses on PORT for TETs concluded that current evidence did not support any benefit of PORT on recurrence in patients with complete resection of stage II or III TETs (42,43). Two meta-analyses, one with 3,823 patients from fourteen studies and the other with 4,746 patients from five studies, showed that increased OS was observed in the subgroup analysis of completely resected stage II or III thymoma (44,45). Hamaji et al. also showed that PORT improves the long-term survival outcomes of patients with thymic carcinoma, although stage-specific or resectional status-specific recommendations were not available in the meta-analysis (46).
These discrepancies among large database studies or meta-analyses can be attributed to the rarity of TETs and the inherent bias in studies, such as patient eligibility, lack of or missing covariates derived from the database, adjuvant radiotherapy, including concurrent use of chemotherapy, or the covariates utilized for propensity score matching analysis. Furthermore, information from Masaoka or Masaoka-Koga staging is not specifically recorded in the NCDB or the SEER database and is inferred and subjectively classified based on recorded information as far as possible. This could be a limitation of large database analysis (Table 4).
Table 4
| Database | Pros | Cons |
|---|---|---|
| All | Large series of patients | Retrospective studies contain bias; no detailed records of failure patterns, chemotherapy, and radiotherapy |
| SEER | Propensity score-matched studies were performed; cause of death and second malignancy were available; can be focused on specific histological type | Surgical margin status, comorbidities, patient performance status, and Masaoka-Koga staging were unavailable; WHO histology classification was unavailable for the majority; no central review of histological classification was performed; the ethnic characteristics were diverse |
| ChART | Propensity score-matched studies were performed; TNM staging was utilized | Missing patient information caused the majority to excluded from the analysis; Masaoka-Koga staging was unavailable |
| JART | The number of patients with missing data (including OS) was very small; Masaoka staging was utilized | No central review of histological classification was performed |
| ESTS | Clinico-pathological variables affecting long-term survival were investigated; Masaoka staging was utilized; neuroendocrine thymic tumors were excluded from the analysis | Only surgical cases in high-volume centers were included; no central review of histological classification was performed; nodal status and the site of distant metastases were unavailable |
| NCDB | Margin status was incorporated into the analysis | Masaoka-Koga staging was unavailable; no central review of histological classification was performed |
| BCCAR | Variability in clinical behavior and practice variations were focused; Masaoka-Koga staging was utilized; pathology review and reclassification were performed | 10% of the data were unavailable for analysis; the number of patients with stage I was limited |
| ITMIG | Completely resected stage II and III thymoma were the focus; Masaoka or Masaoka-Koga staging was utilized | Stages IIA, IIB, and II were all categorized as stage II. |
SEER, Surveillance, Epidemiology, and End Results database; WHO, World Health Organization; ChART, Chinese Alliance for Research of Thymoma database; JART, Japanese Association for Research on the Thymus Database; OS, overall survival; ESTS, European Society of Thoracic Surgeons database; NCDB, National Cancer Database; BCCAR, British Columbia Cancer Agency Registry; ITMIG, International Thymic Malignancies Interest Group Retrospective Database.
The incompleteness of the traditional Masaoka-Koga staging is also a limitation, as it provides no information on the number of involved organs or tumor size, both of which appear to be promising factors for prognostic stratification (17,47). Therefore, an optimal staging system to identify patients with poor prognosis who are at high risk for recurrence is necessary. Such patients could be ideal candidates for PORT or less-invasive surgery combined with PORT. For example, in patients with phrenic nerve involvement (Masaoka-Koga stage III or higher), en bloc resection can lead to diaphragmatic impairment and pulmonary function deterioration. Phrenic nerve-sparing surgery combined with PORT is feasible with an acceptable local control rate of 92.9% (48). The ninth edition of the TNM stage classification, which is based on a large international database, is expected to contribute significantly to this field.
WHO histological subtypes
The patterns of metastasis and recurrence significantly differ across the WHO classification histological subtypes; the time to metastasis is shortest in thymic carcinoma, followed by high-risk thymoma (WHO types B2 and B3), and longest in low-risk thymoma (A, AB, and B1) (9,12,49,50). According to an analysis of the ITMIG retrospective database, PORT was associated with a trend toward better OS in all subgroups of stage II and III thymoma, and the greatest and most statistically significant survival advantage with PORT was observed in the subgroup of patients with stage III WHO types B1, B2, or B3 thymoma (29). In contrast, Muslim et al. reported that SEER database analysis did not reveal a significant disease-specific survival advantage of PORT in any of the WHO histological subtypes among patients with stage IIB–IV thymoma (38). This could be mainly due to differences in the stages of the eligible patient cohorts. For thymic carcinoma, we did not find any reports examining the differences between squamous cell carcinoma and adenocarcinoma.
Resectional status
Complete resection was associated with improved OS in patients with TETs. Resectional status is a well-known factor for indicating PORT in TETs because PORT is associated with improved OS in patients with incomplete resections or positive margins (28,30,35).
When total resection is not possible, subtotal resection, or debulking surgery, may yield a higher survival rate than that for inoperable thymoma but not for thymic carcinoma (5). Zhai et al. conducted a retrospective study on debulking surgery plus PORT versus radiotherapy in 47 patients with unresectable stage III thymic carcinoma (51). The results revealed 5-year OS rates of 54.4% and 0%, respectively. Thus, there may be merit in the so-called “debulking procedures” followed by PORT, but only in highly selected cases. Mastromarino et al. investigated 79 patients with types B2 and B3 thymomas, including R1 or R2 residual tumors (52). Regardless of whether residual tumors existed in the primary tumor or pleural space, PORT significantly improved progression-free survival in patients with R1 residual tumors, whereas postoperative chemotherapy or chemoradiotherapy improved cancer-specific survival in patients with R2 residual tumors.
In summary, the current literature suggests that PORT does not improve OS in stage I–IIA TETs, with inconsistent results for stage IIB–III TETs. The currently available data suggest that stage II–III TETs are a heterogeneous population; at least stages IIA and IIB need to be considered separately as indications for PORT, not considered together as stage II. PORT for stage III TETs can contribute to improved OS in patients with higher-risk grades, such as carcinoma or WHO type B2–B3, and may benefit from PORT in terms of improved OS when they do not develop distant metastasis. Identifying patients less likely to develop early distant metastases and can genuinely benefit from PORT remains a gray zone that requires further exploration.
Recurrent TET
After definitive radiotherapy or PORT, the 5-year cumulative incidence of all intrathoracic failures was 24% (53). The most common site of failure was the out-of-field pleural space, followed by the 5-year incidence of in-field failure of 7%. Although radiotherapy is critical in the multimodal treatment of intrathoracic recurrent TETs (54,55), several aspects warrant careful consideration.
First, the prognostic significance of PORT in patients with recurrent TETs remains unclear. Several studies with a limited sample size have indicated that adjuvant therapies, including PORT, do not effectively reduce recurrence or improve survival outcomes (56,57). Second, when considering PORT for recurrent TETs, examining the overlap between the field irradiated during initial PORT and the target volume at the time of recurrence is imperative. Furthermore, it is essential to ensure that the cumulative dose to the organs at risk is within the established dose constraints, as described in “Radiotherapy techniques”. Recently, phase II trials have demonstrated that targeted therapies, including everolimus, lenvatinib, and sunitinib, may induce durable disease control in patients with recurrent TETs as second-line treatment (58-60). However, there is no evidence supporting the concurrent use of systemic therapies and radiotherapy. Therefore, when administering PORT for recurrent TETs, pausing targeted therapy before and after PORT based on its half-life should be considered.
Radiotherapy techniques
Photon beam radiotherapy
Photon beam radiotherapy is commonly used in PORT. In this section, we outline the standard procedures and techniques for photon beam radiotherapy based on previous literature and guidelines (13,61,62). Three-dimensional conformal radiotherapy (3D-CRT) is conventionally used as a common delivery technique. Recently, intensity-modulated radiotherapy (IMRT), which enables the delivery of conformal radiation doses to irregularly shaped target volumes with high-precision fitted dose distribution, could be expected to decrease the dose delivered to organs at risk, sparing over 3D-CRT and has been applied to PORT (63).
Before initiating PORT, physicians should ensure the absence of infection or wound dehiscence. While there is no definitive maximum period from surgery to PORT, it has been reported that a delay of more than 3 months post-surgery often leads physicians to decide to skip PORT (14). Given that the respiratory motion of the upper mediastinum is typically minimal, respiratory-gated radiotherapy or breath-hold radiotherapy techniques to reduce motion are not mandatory.
Postoperative changes should be considered when delineating target volumes. As expert agreement for delineating postoperative cases is low compared with that of definitive cases, a contouring atlas for TETs with expert consensus is urgently needed (64). The utilization of four-dimensional-CT and positron emission tomography-CT fusion should be implemented for contour delineation, if available (64). It is also recommended to fuse preoperative CT with treatment-planning CT and deform preoperative CT to fit the treatment-planning CT. Gross tumor volume was defined as incomplete resection. In cases of complete resection, the clinical target volume (CTV) should encompass the tumor bed, surgical clips, and potential sites of residual disease. In cases of incomplete resection, the entire thymus should be included in the CTV. In instances where the margins are close or positive, surgical clips are useful for identifying the site of boost irradiation. The rates of lymphogenous metastasis in thymoma and thymic carcinoma are 1.8 and 27%, respectively (65). There was no significant difference in 5-year OS between local radiation therapy (targeting the tumor bed and anterior mediastinal areas only) and elective nodal irradiation (targeting the entire mediastinal and supraclavicular regions) in patients with TETs (65,66). Therefore, elective nodal irradiation is not recommended for TETs. The prescribed doses range from 45–50 Gy for negative or close margins, 54 Gy for microscopically positive resection margins, and 60–70 Gy for gross residual disease, administered in 1.8–2.0 Gy fractions.
As the dose constraints of organs at risk, normal tissue dose-volume constraints for conventionally fractionated radiotherapy for lung cancer could be applied to PORT in TETs; spinal cord max dose ≤50 Gy; lung V20Gy ≤35–40% (VxGy: percentage of the volume receiving at least X Gy), mean dose ≤20 Gy; heart V50Gy ≤25%, mean dose ≤20 Gy; esophagus mean dose ≤34 Gy, V60Gy ≤17% (67).
Proton beam therapy
A dosimetric comparison study has shown that both proton beam radiotherapy and carbon-ion radiotherapy excel in sparing organs at risk, such as the heart, lungs, left ventricle, esophagus, and spinal cord, and in improving target volume coverage when compared to photon IMRT (63,68-72). This is anticipated to reduce toxicity, potentially decreasing major cardiac events and the occurrence of secondary malignant neoplasms. Previous studies on proton beam radiotherapy for TETs are presented in Table 5. No toxicities more severe than grade 3 were observed following proton beam radiotherapy, and the OS and locoregional control rates were comparable with those achieved with photon beam radiotherapy. It should be noted that the sample sizes in these studies were small and included both PORT and definitive RT. Future clinical trials with larger cohorts and direct comparisons between proton and photon beam radiotherapies are essential.
Table 5
| Authors | Year | N [N of PORT] | Primary | Prescribed dose, median (range) | Efficacy | Toxicity ≥ grade 3 |
|---|---|---|---|---|---|---|
| Vogel et al. (73) | 2016 | 27 [17] | TET | 61.2 CGE (50.4–70.0 CGE) | 3-year OS: 94% | No |
| 3-year regional control: 96% | ||||||
| Parikh et al. (71) | 2016 | 4 [4] | Thymoma | 57.0 CGE (50.4–66.6 CGE) | No death and no recurrences occurred | No |
| Zhu et al. (70) | 2018 | 6 [5] | Thymoma | 60 GyE (54–70 GyE) | 3 patients experienced recurrences (0 local recurrence) | No |
| Mercado et al. (74) | 2019 | 30 [26] | TET | 54 GyE (45–70 GyE) | 5 patients experienced recurrence (1 local recurrence) | No |
| 4 died (3 died of TETs) |
TET, thymic epithelial tumor; PORT, postoperative radiotherapy; CGE, cobalt-gray equivalent; OS, overall survival; GyE, gray equivalent.
Toxicity
Common acute toxicities associated with PORT for TETs include fatigue, dermatitis, esophagitis, pneumonitis, and myelosuppression. Given the location of the anterior mediastinum, the severity of esophagitis is typically milder than in other intrathoracic tumors such as esophageal or lung cancers. Consequently, this study focused on detailing the more significant late toxicities that require special consideration, including pneumonitis, cardiotoxicities, and secondary malignancies.
Radiation pneumonitis
Previous studies have reported that the irradiated dose to the normal lung is one of the common risk factors for developing radiation pneumonitis in patients with lung cancer: lung V20Gy, mean lung dose, and absolute lung volume spared from a 5 Gy dose (75,76). In PORT for TETs, the irradiated dose to the normal lung is usually lower than that in definitive radiotherapy for lung cancer because the tumor bed is located in the mediastinum. The incidence of grade 2 or higher radiation pneumonitis in PORT for TETs is reported to be <10%, which is lower than that in definitive radiotherapy for lung cancer. Therefore, only a few studies have reported the risk factors for radiation pneumonitis in TETs. Moiseenko et al. quantified the influence of irradiated lung volume and dose on the lung response and showed that the mean lung dose was strongly correlated with lung complications, including pneumonitis and fibrosis (77). Tomita et al. reported that pulmonary artery V35Gy was significantly associated with radiation pneumonitis in patients with TET (78). In summary, efforts should be made to reduce the irradiation dose to the normal lung as much as possible during PORT for TETs, even though the risk of radiation pneumonitis is very low.
Radiation-induced cardiotoxicities
The onset of radiation-induced cardiotoxicities occurs years or decades after PORT, and the typical symptoms are acute pericarditis, pericardial effusion, coronary artery disease, stenosis and regurgitation of valves, arrhythmia, and heart failure. Radiation-induced cardiotoxicities are a concern in long-term survivors of thoracic irradiation, such as patients with breast cancer or lymphoma. It is well known that after PORT in patients with breast cancer, the rates of major coronary events increase linearly with the mean dose to the heart by 7.4% per gray (79). Meanwhile, a SEER database analysis showed that radiotherapy does not increase the risk of cardiac mortality (12-year cumulative incidence or death, 10.2% radiation vs. 7.5% no radiation) in patients with thymoma (18). Given the prolonged survival of patients undergoing TETs, cardiotoxicity remains a critical concern following mediastinal irradiation via PORT. There is a pressing need for prospective studies that screen for cardiac event risks and consistently monitor radiation-induced cardiotoxicity.
Second malignancies
Thymomas are associated with an increased risk of secondary malignancies. Patients face a higher risk of death from this second type of cancer than from recurrence (80). The lifetime attributable risk of secondary fatal cancer in patients receiving PORT (50 Gy in 25 fractions) for thymoma has been reported to be approximately 1–3% (81). Mou et al. reported that patients with thymoma who underwent surgery with PORT had a higher rate of secondary cancers than those who underwent surgery without PORT, based on the SEER database (32). In contrast, two studies concluded that radiotherapy did not increase the risk of secondary malignancy in patients with thymoma (18,82).
The incidence of secondary malignancies and deaths from secondary malignancies are lower in thymic carcinoma than in thymoma (83).
The association between TETs and secondary malignancies cannot be attributed solely to radiotherapy. Further investigations with long-term follow-ups and large sample sizes are needed because of the rare incidence of TETs and secondary malignancies.
Future indications
Radiotherapy has undergone significant advancements in recent decades. Research supporting the use of IMRT or particle therapy is ongoing, and accumulated data are expected to be published in the near future, contributing to the establishment of new evidence. Adopting these innovative radiotherapy techniques for TETs is expected to enhance patient outcomes (84). For definitive treatment or PORT, we have highlighted the radiotherapy techniques projected to be utilized for TETs in the coming years.
RADIORHYTHMIC, a phase III randomized study of PORT in stage IIB/III thymomas after complete surgical resection, was conducted by the RYTHMIC and is currently ongoing (85). Three hundred and fourteen patients will be randomized to either the PORT group (50–54 Gy to the mediastinum using IMRT or proton beam therapy) or the surveillance group. The results will be expected in 2028. Hemithoracic intensity-modulated pleural radiation therapy (IMPRINT) for malignant pleural mesothelioma has been developed as part of a multimodality treatment for patients receiving pleurectomy/decortication to spare the affected side of the lung (86). As recurrent TETs often develop pleural dissemination, IMPRINT can be applied to control pleural dissemination (53,87). SABR-COMET, a randomized phase II study aimed at determining the effect of stereotactic body radiotherapy (SBRT) in patients with a controlled primary tumor and 1–5 oligometastatic lesions, demonstrated that SBRT was associated with improved OS (88). Yano et al. reported 24 patients with recurrent thymoma, revealing that patients with a limited number of recurrent lesions had a better prognosis regardless of treatment (89). Based on these findings, SBRT may be an effective treatment option for patients with oligometastatic TETs. Adaptive radiotherapy, which aims to decrease the dose to normal tissues and allow for dose escalation to the target volume by changing the radiation treatment plan delivered to a patient during the course of radiotherapy to account for either temporal changes in anatomy (e.g., tumor size, internal motion, variations in respiratory patterns, weight loss) or changes in tumor biology/function (e.g., hypoxia) (90), is promising for application in TETs (91,92). Intraoperative radiotherapy has been used for intractable cancers such as pancreatic cancer and osteosarcoma (93,94). Cui et al. applied intraoperative radiotherapy (8–10 Gy) to TETs and reported its safety and efficacy in 14 patients with invasive thymomas as a less time-consuming and less invasive radiotherapy technique for improving locoregional control (95). The mean time for installation and operation of the radiation equipment 57.6 minutes (range, 48–72 minutes). During a median follow-up period of 41 months, no recurrence, death, or severe toxicity were observed.
As part of the multimodal treatment, surgical approaches, systemic therapy, and radiotherapy techniques are drastically evolving. Salfity et al. reported on minimally invasive surgery in managing resectable thymomas based on the NCDB and showed that PORT was less frequent in thoracoscopic thymectomies than in traditional open sternotomy (96). As the efficacy of PORT in different surgical approaches remains unknown, future investigations may reveal the different indications and irradiation fields for PORT depending on the surgical approach. Several studies have reported abscopal and bystander effects after radiotherapy in thymic carcinoma (97,98). A combination of immunotherapy and radiotherapy is expected to enhance these effects and improve patient outcomes. The abscopal effect was proposed in 1953, and it is hypothesized that the immune system plays a role in mediating this phenomenon, leading to therapeutic effects on lesions located outside the irradiated field (99,100). Currently, a clinical investigation on the abscopal effect of radiotherapy in combination with recombinant human granulocyte-macrophage colony-stimulating factor for advanced TETs is ongoing in China (ClinicalTrials.gov; NCT05407649).
Advancements in radiotherapy techniques combined with other novel surgical or systematic approaches can further improve the outcomes. Therefore, an optimal treatment strategy combined with PORT should be identified using prospective data.
Conclusions
This narrative review presents a synthesis of the existing literature on the efficacy and toxicities of PORT in relation to OS. Considering the Masaoka-Koga staging, WHO histological subtypes, and resection status, indications for PORT have been determined for stage IIB–III TETs, although inconsistent results have been observed. Identifying patients who benefit from PORT for locoregional control can refine treatment strategies for TETs. Given that TETs typically result in long-term survival, late toxicities, such as radiation pneumonitis, radiation-induced cardiac toxicities, and secondary malignancies, are significant. However, a decrease in these toxicities has been anticipated with the introduction of advanced radiotherapy techniques. Further studies are required to evaluate the value of PORT based on patient characteristics and combination therapy.
Acknowledgments
Funding: This work was supported by
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Masatsugu Hamaji) for the series “Locally Advanced Thymic Epithelial Tumors” published in Mediastinum. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://med.amegroups.com/article/view/10.21037/med-23-38/rc
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-23-38/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-23-38/coif). The series “Locally Advanced Thymic Epithelial Tumors” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Engels EA, Pfeiffer RM. Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer 2003;105:546-51. [Crossref] [PubMed]
- de Jong WK, Blaauwgeers JL, Schaapveld M, et al. Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur J Cancer 2008;44:123-30. [Crossref] [PubMed]
- Zhao Y, Shi J, Fan L, et al. Surgical treatment of thymoma: an 11-year experience with 761 patients. Eur J Cardiothorac Surg 2016;49:1144-9. [Crossref] [PubMed]
- Litvak AM, Woo K, Hayes S, et al. Clinical characteristics and outcomes for patients with thymic carcinoma: evaluation of Masaoka staging. J Thorac Oncol 2014;9:1810-5. [Crossref] [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. [Crossref] [PubMed]
- Eng TY, Fuller CD, Jagirdar J, et al. Thymic carcinoma: state of the art review. Int J Radiat Oncol Biol Phys 2004;59:654-64. [Crossref] [PubMed]
- Koga K, Matsuno Y, Noguchi M, et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int 1994;44:359-67. [Crossref] [PubMed]
- Detterbeck FC, Nicholson AG, Kondo K, et al. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. J Thorac Oncol 2011;6:S1710-6. [Crossref] [PubMed]
- Marx A, Chan JKC, Chalabreysse L, et al. The 2021 WHO Classification of Tumors of the Thymus and Mediastinum: What Is New in Thymic Epithelial, Germ Cell, and Mesenchymal Tumors? J Thorac Oncol 2022;17:200-13. [Crossref] [PubMed]
- Girard N, Mornex F, Van Houtte P, et al. Thymoma: a focus on current therapeutic management. J Thorac Oncol 2009;4:119-26. [Crossref] [PubMed]
- Gao L, Wang C, Fang W, et al. Outcome of multimodality treatment for 188 cases of type B3 thymoma. J Thorac Oncol 2013;8:1329-34. [Crossref] [PubMed]
- Weis CA, Yao X, Deng Y, et al. The impact of thymoma histotype on prognosis in a worldwide database. J Thorac Oncol 2015;10:367-72. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Thymomas and Thymic Carcinomas (Version 1.2023); (cited 2023 Aug 3). Available online: https://www.nccn.org/professionals/physician_gls/pdf/thymic.pdf
- Basse C, Thureau S, Bota S, et al. Multidisciplinary Tumor Board Decision Making for Postoperative Radiotherapy in Thymic Epithelial Tumors: Insights from the RYTHMIC Prospective Cohort. J Thorac Oncol 2017;12:1715-22. [Crossref] [PubMed]
- Ahmad U. The eighth edition TNM stage classification for thymic tumors: What do I need to know? J Thorac Cardiovasc Surg 2021;161:1524-9.
- Liang G, Gu Z, Li Y, et al. Comparison of the Masaoka-Koga staging and the International Association for the Study of Lung Cancer/the International Thymic Malignancies Interest Group proposal for the TNM staging systems based on the Chinese Alliance for Research in Thymomas retrospective database. J Thorac Dis 2016;8:727-37. [Crossref] [PubMed]
- Chiappetta M, Lococo F, Pogliani L, et al. Masaoka-Koga and TNM Staging System in Thymic Epithelial Tumors: Prognostic Comparison and the Role of the Number of Involved Structures. Cancers (Basel) 2021;13:5254. [Crossref] [PubMed]
- Fernandes AT, Shinohara ET, Guo M, et al. The role of radiation therapy in malignant thymoma: a Surveillance, Epidemiology, and End Results database analysis. J Thorac Oncol 2010;5:1454-60. [Crossref] [PubMed]
- Patel S, Macdonald OK, Nagda S, et al. Evaluation of the role of radiation therapy in the management of malignant thymoma. Int J Radiat Oncol Biol Phys 2012;82:1797-801. [Crossref] [PubMed]
- Weksler B, Shende M, Nason KS, et al. The role of adjuvant radiation therapy for resected stage III thymoma: a population-based study. Ann Thorac Surg 2012;93:1822-8; discussion 1828-9. [Crossref] [PubMed]
- Mariano C, Ionescu DN, Cheung WY, et al. Thymoma: a population-based study of the management and outcomes for the province of British Columbia. J Thorac Oncol 2013;8:109-17. [Crossref] [PubMed]
- Ruffini E, Detterbeck F, Van Raemdonck D, et al. Thymic carcinoma: a cohort study of patients from the European society of thoracic surgeons database. J Thorac Oncol 2014;9:541-8. [Crossref] [PubMed]
- Omasa M, Date H, Sozu T, et al. Postoperative radiotherapy is effective for thymic carcinoma but not for thymoma in stage II and III thymic epithelial tumors: the Japanese Association for Research on the Thymus Database Study. Cancer 2015;121:1008-16. [Crossref] [PubMed]
- Hishida T, Nomura S, Yano M, et al. Long-term outcome and prognostic factors of surgically treated thymic carcinoma: results of 306 cases from a Japanese Nationwide Database Study. Eur J Cardiothorac Surg 2016;49:835-41. [Crossref] [PubMed]
- Lim YJ, Kim HJ, Wu HG. Role of Postoperative Radiotherapy in Nonlocalized Thymoma: Propensity-Matched Analysis of Surveillance, Epidemiology, and End Results Database. J Thorac Oncol 2015;10:1357-63. [Crossref] [PubMed]
- Fu H, Gu ZT, Fang WT, et al. Long-Term Survival After Surgical Treatment of Thymic Carcinoma: A Retrospective Analysis from the Chinese Alliance for Research of Thymoma Database. Ann Surg Oncol 2016;23:619-25. [Crossref] [PubMed]
- Wang F, Pang L, Fu J, et al. Postoperative survival for patients with thymoma complicating myasthenia gravis-preliminary retrospective results of the ChART database. J Thorac Dis 2016;8:711-7. [Crossref] [PubMed]
- Liu Q, Gu Z, Yang F, et al. The role of postoperative radiotherapy for stage I/II/III thymic tumor-results of the ChART retrospective database. J Thorac Dis 2016;8:687-95. [Crossref] [PubMed]
- Rimner A, Yao X, Huang J, et al. Postoperative Radiation Therapy Is Associated with Longer Overall Survival in Completely Resected Stage II and III Thymoma-An Analysis of the International Thymic Malignancies Interest Group Retrospective Database. J Thorac Oncol 2016;11:1785-92. [Crossref] [PubMed]
- Jackson MW, Palma DA, Camidge DR, et al. The Impact of Postoperative Radiotherapy for Thymoma and Thymic Carcinoma. J Thorac Oncol 2017;12:734-44. [Crossref] [PubMed]
- Lim YJ, Song C, Kim JS. Improved survival with postoperative radiotherapy in thymic carcinoma: A propensity-matched analysis of Surveillance, Epidemiology, and End Results (SEER) database. Lung Cancer 2017;108:161-7. [Crossref] [PubMed]
- Mou H, Liao Q, Hou X, et al. Clinical characteristics, risk factors, and outcomes after adjuvant radiotherapy for patients with thymoma in the United States: analysis of the Surveillance, Epidemiology, and End Results (SEER) Registry (1988-2013). Int J Radiat Biol 2018;94:495-502. [Crossref] [PubMed]
- Bian D, Zhou F, Yang W, et al. Thymoma size significantly affects the survival, metastasis and effectiveness of adjuvant therapies: a population based study. Oncotarget 2018;9:12273-83. [Crossref] [PubMed]
- Gu Z, Chen C, Wang Y, et al. Video-assisted thoracoscopic surgery versus open surgery for Stage I thymic epithelial tumours: a propensity score-matched study. Eur J Cardiothorac Surg 2018;54:1037-44. [Crossref] [PubMed]
- Kim S, Bull DA, Hsu CH, et al. The Role of Adjuvant Therapy in Advanced Thymic Carcinoma: A National Cancer Database Analysis. Ann Thorac Surg 2020;109:1095-103. [Crossref] [PubMed]
- Mou H, Kong Y, Wu Y, et al. Effect of Postoperative Radiotherapy in Thymoma Patients: A SEER-Based Study. Oncol Res Treat 2021;44:28-35. [Crossref] [PubMed]
- Wu J, Wang Z, Jing C, et al. The incidence and prognosis of thymic squamous cell carcinoma: A Surveillance, Epidemiology, and End Results Program population-based study. Medicine (Baltimore) 2021;100:e25331. [Crossref] [PubMed]
- Muslim Z, Baig MZ, Weber JF, et al. Invasive thymoma - Which patients benefit from post-operative radiotherapy? Asian Cardiovasc Thorac Ann 2021;29:935-42. [Crossref] [PubMed]
- Lococo F, Nachira D, Chiappetta M, et al. Does Myasthenia Gravis Affect Long-Term Survival in Thymic Carcinomas? An ESTS Database Analysis. Diagnostics (Basel) 2022;12:1764. [Crossref] [PubMed]
- Zhang C, Wang Q, Hu L, et al. The Prognostic Value of Postoperative Radiotherapy for Thymoma and Thymic Carcinoma: A Propensity-Matched Study Based on SEER Database. Cancers (Basel) 2022;14:4938. [Crossref] [PubMed]
- Lin LM, Li YM, Huang YX, et al. Evaluation of the role of postoperative radiotherapy in locally invasive thymoma: A propensity-matched study based on the SEER database. PLoS One 2023;18:e0283192. [Crossref] [PubMed]
- Ma J, Sun X, Huang L, et al. Postoperative radiotherapy and tumor recurrence after complete resection of stage II/III thymic tumor: a meta-analysis of cohort studies. Onco Targets Ther 2016;9:4517-26. [Crossref] [PubMed]
- Korst RJ, Kansler AL, Christos PJ, et al. Adjuvant radiotherapy for thymic epithelial tumors: a systematic review and meta-analysis. Ann Thorac Surg 2009;87:1641-7. [Crossref] [PubMed]
- Zhou D, Deng XF, Liu QX, et al. The Effectiveness of Postoperative Radiotherapy in Patients With Completely Resected Thymoma: A Meta-Analysis. Ann Thorac Surg 2016;101:305-10. [Crossref] [PubMed]
- Tateishi Y, Horita N, Namkoong H, et al. Postoperative Radiotherapy for Completely Resected Masaoka/Masaoka-Koga Stage II/III Thymoma Improves Overall Survival: An Updated Meta-Analysis of 4746 Patients. J Thorac Oncol 2021;16:677-85. [Crossref] [PubMed]
- Hamaji M, Shah RM, Ali SO, et al. A Meta-Analysis of Postoperative Radiotherapy for Thymic Carcinoma. Ann Thorac Surg 2017;103:1668-75. [Crossref] [PubMed]
- Rimner A, Ruffini E, Cilento V, et al. The International Association for the Study of Lung Cancer Thymic Epithelial Tumors Staging Project: An Overview of the Central Database Informing Revision of the Forthcoming (Ninth) Edition of the TNM Classification of Malignant Tumors. J Thorac Oncol 2023;18:1386-98. [Crossref] [PubMed]
- Aprile V, Bertoglio P, Korasidis S, et al. Nerve-Sparing Surgery in Advanced Stage Thymomas. Ann Thorac Surg 2019;107:878-84. [Crossref] [PubMed]
- Khandelwal A, Sholl LM, Araki T, et al. Patterns of metastasis and recurrence in thymic epithelial tumours: longitudinal imaging review in correlation with histological subtypes. Clin Radiol 2016;71:1010-7. [Crossref] [PubMed]
- Huang J, Rizk NP, Travis WD, et al. Comparison of patterns of relapse in thymic carcinoma and thymoma. J Thorac Cardiovasc Surg 2009;138:26-31. [Crossref] [PubMed]
- Zhai Y, Hui Z, Gao Y, et al. Debulking Surgery Plus Radiation: Treatment Choice for Unresectable Stage III Thymic Carcinoma. Thorac Cardiovasc Surg 2020;68:440-5. [Crossref] [PubMed]
- Mastromarino MG, Bacchin D, Aprile V, et al. Unradical Surgery for Locally-Advanced Thymoma: Is it time to evolve Perspectives? Lung Cancer 2023;180:107214. [Crossref] [PubMed]
- Rimner A, Gomez DR, Wu AJ, et al. Failure patterns relative to radiation treatment fields for stage II-IV thymoma. J Thorac Oncol 2014;9:403-9. [Crossref] [PubMed]
- Carretta A, Ciriaco P, Muriana P, et al. Surgical treatment of single and multiple thymoma recurrences. Gen Thorac Cardiovasc Surg 2020;68:350-6. [Crossref] [PubMed]
- Ciccone AM, Rendina EA. Treatment of recurrent thymic tumors. Semin Thorac Cardiovasc Surg 2005;17:27-31. [Crossref] [PubMed]
- Haniuda M, Kondo R, Numanami H, et al. Recurrence of thymoma: clinicopathological features, re-operation, and outcome. J Surg Oncol 2001;78:183-8. [Crossref] [PubMed]
- Sandri A, Cusumano G, Lococo F, et al. Long-term results after treatment for recurrent thymoma: a multicenter analysis. J Thorac Oncol 2014;9:1796-804. [Crossref] [PubMed]
- Zucali PA, De Pas T, Palmieri G, et al. Phase II Study of Everolimus in Patients With Thymoma and Thymic Carcinoma Previously Treated With Cisplatin-Based Chemotherapy. J Clin Oncol 2018;36:342-9. [Crossref] [PubMed]
- Sato J, Satouchi M, Itoh S, et al. Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): a multicentre, phase 2 trial. Lancet Oncol 2020;21:843-50. [Crossref] [PubMed]
- Thomas A, Rajan A, Berman A, et al. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: an open-label phase 2 trial. Lancet Oncol 2015;16:177-86. [Crossref] [PubMed]
- Girard N, Ruffini E, Marx A, et al. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v40-55. [Crossref] [PubMed]
- Chun SG, Rimner A, Amini A, et al. American Radium Society Appropriate Use Criteria for Radiation Therapy in the Multidisciplinary Management of Thymic Carcinoma. JAMA Oncol 2023;9:971-80. [Crossref] [PubMed]
- Haefner MF, Verma V, Bougatf N, et al. Dosimetric comparison of advanced radiotherapy approaches using photon techniques and particle therapy in the postoperative management of thymoma. Acta Oncol 2018;57:1713-20. [Crossref] [PubMed]
- Holliday E, Fuller CD, Kalpathy-Cramer J, et al. Quantitative assessment of target delineation variability for thymic cancers: Agreement evaluation of a prospective segmentation challenge. J Radiat Oncol 2016;5:55-61. [Crossref] [PubMed]
- Kondo K, Monden Y. Lymphogenous and hematogenous metastasis of thymic epithelial tumors. Ann Thorac Surg 2003;76:1859-64; discussion 1864-5. [Crossref] [PubMed]
- Kim YJ, Kim SS, Song SY, et al. Elective Nodal Irradiation as Adjuvant Radiotherapy for Advanced Thymomas and Thymic Carcinomas. Clin Lung Cancer 2019;20:e91-6. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Non-small cell lung cancer (Version 3.2023). Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Vogel J, Lin L, Simone CB 2nd, et al. Risk of major cardiac events following adjuvant proton versus photon radiation therapy for patients with thymic malignancies. Acta Oncol 2017;56:1060-4. [Crossref] [PubMed]
- Vogel J, Lin L, Litzky LA, et al. Predicted Rate of Secondary Malignancies Following Adjuvant Proton Versus Photon Radiation Therapy for Thymoma. Int J Radiat Oncol Biol Phys 2017;99:427-33. [Crossref] [PubMed]
- Zhu HJ, Hoppe BS, Flampouri S, et al. Rationale and early outcomes for the management of thymoma with proton therapy. Transl Lung Cancer Res 2018;7:106-13. [Crossref] [PubMed]
- Parikh RR, Rhome R, Hug E, et al. Adjuvant Proton Beam Therapy in the Management of Thymoma: A Dosimetric Comparison and Acute Toxicities. Clin Lung Cancer 2016;17:362-6. [Crossref] [PubMed]
- Franceschini D, Cozzi L, Loi M, et al. Volumetric modulated arc therapy versus intensity-modulated proton therapy in the postoperative irradiation of thymoma. J Cancer Res Clin Oncol 2020;146:2267-76. [Crossref] [PubMed]
- Vogel J, Berman AT, Lin L, et al. Prospective study of proton beam radiation therapy for adjuvant and definitive treatment of thymoma and thymic carcinoma: Early response and toxicity assessment. Radiother Oncol 2016;118:504-9. [Crossref] [PubMed]
- Mercado CE, Hartsell WF, Simone CB 2nd, et al. Proton therapy for thymic malignancies: multi-institutional patterns-of-care and early clinical outcomes from the proton collaborative group and the university of Florida prospective registries. Acta Oncol 2019;58:1036-40. [Crossref] [PubMed]
- Tsujino K, Hashimoto T, Shimada T, et al. Combined analysis of V20, VS5, pulmonary fibrosis score on baseline computed tomography, and patient age improves prediction of severe radiation pneumonitis after concurrent chemoradiotherapy for locally advanced non-small-cell lung cancer. J Thorac Oncol 2014;9:983-90. [Crossref] [PubMed]
- Barriger RB, Fakiris AJ, Hanna N, et al. Dose-volume analysis of radiation pneumonitis in non-small-cell lung cancer patients treated with concurrent cisplatinum and etoposide with or without consolidation docetaxel. Int J Radiat Oncol Biol Phys 2010;78:1381-6. [Crossref] [PubMed]
- Moiseenko V, Craig T, Bezjak A, et al. Dose-volume analysis of lung complications in the radiation treatment of malignant thymoma: a retrospective review. Radiother Oncol 2003;67:265-74. [Crossref] [PubMed]
- Tomita N, Okuda K, Ogawa Y, et al. Relationship between radiation doses to heart substructures and radiation pneumonitis in patients with thymic epithelial tumors. Sci Rep 2020;10:11191. [Crossref] [PubMed]
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987-98. [Crossref] [PubMed]
- Hamaji M, Sozu T, Machida R, et al. Second malignancy versus recurrence after complete resection of thymoma. Asian Cardiovasc Thorac Ann 2018;26:290-5. [Crossref] [PubMed]
- Jalbout W, Jbara R, Rizk C, et al. On the risk of secondary cancer from thymoma radiotherapy. Phys Med Biol 2022; [Crossref] [PubMed]
- Pan CC, Chen PC, Wang LS, et al. Thymoma is associated with an increased risk of second malignancy. Cancer 2001;92:2406-11. [Crossref] [PubMed]
- Hamaji M, Kawaguchi A, Omasa M, et al. Low incidence of and mortality from a second malignancy after resection of thymic carcinoma†. Interact Cardiovasc Thorac Surg 2019;28:375-9. [Crossref] [PubMed]
- Roden AC, Ahmad U, Cardillo GThymic Carcinomas-A Concise Multidisciplinary Update on Recent Developments From the Thymic Carcinoma Working Group of the International Thymic Malignancy Interest Group, et al. J Thorac Oncol 2022;17:637-50. [Crossref] [PubMed]
- Basse C, Botticella A, Molina TJ, et al. RADIORYTHMIC: Phase III, Opened, Randomized Study of Postoperative Radiotherapy Versus Surveillance in Stage IIb/III of Masaoka Koga Thymoma after Complete Surgical Resection. Clin Lung Cancer 2021;22:469-72. [Crossref] [PubMed]
- Rimner A, Zauderer MG, Gomez DR, et al. Phase II Study of Hemithoracic Intensity-Modulated Pleural Radiation Therapy (IMPRINT) As Part of Lung-Sparing Multimodality Therapy in Patients With Malignant Pleural Mesothelioma. J Clin Oncol 2016;34:2761-8. [Crossref] [PubMed]
- Kamel MK, Stiles BM, Ghaly G, et al. Predictors of Pleural Implants in Patients With Thymic Tumors. Ann Thorac Surg 2016;102:1647-52. [Crossref] [PubMed]
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019;393:2051-8. [Crossref] [PubMed]
- Yano M, Sasaki H, Moriyama S, et al. Number of recurrent lesions is a prognostic factor in recurrent thymoma. Interact Cardiovasc Thorac Surg 2011;13:21-4. [Crossref] [PubMed]
- Schwarz M, Cattaneo GM, Marrazzo L. Geometrical and dosimetrical uncertainties in hypofractionated radiotherapy of the lung: A review. Phys Med 2017;36:126-39. [Crossref] [PubMed]
- Rohrer Bley C, Meier V, Schneider U. Dosimetric benefit of adaptive radiotherapy in the neoadjuvant management of canine and feline thymoma-An exploratory case series. Vet Comp Oncol 2018;16:324-9. [Crossref] [PubMed]
- McCready JE, Poirier VJ, Fleck A, et al. Adaptive radiation therapy using weekly hypofractionation for thymoma treatment: A retrospective study of 10 rabbits. Vet Comp Oncol 2022;20:559-67. [Crossref] [PubMed]
- Kokubo M, Nishimura Y, Shibamoto Y, et al. Analysis of the clinical benefit of intraoperative radiotherapy in patients undergoing macroscopically curative resection for pancreatic cancer. Int J Radiat Oncol Biol Phys 2000;48:1081-7. [Crossref] [PubMed]
- Oya N, Kokubo M, Mizowaki T, et al. Definitive intraoperative very high-dose radiotherapy for localized osteosarcoma in the extremities. Int J Radiat Oncol Biol Phys 2001;51:87-93. [Crossref] [PubMed]
- Cui TX, Dai JG, Li JM, et al. Safety and efficacy of INTRABEAM intraoperative radiotherapy for invasive thymoma. Medicine (Baltimore) 2020;99:e20964. [Crossref] [PubMed]
- Salfity HV, Timsina L, Ceppa DP, et al. Minimally invasive surgery in the management of resectable thymoma: a retrospective analysis from the National Cancer Database. J Thorac Dis 2021;13:6353-62. [Crossref] [PubMed]
- Lesueur P, Chevalier F, Stefan D, et al. Review of the mechanisms involved in the abscopal effect and future directions with a focus on thymic carcinoma. Tumori 2017;103:217-22. [Crossref] [PubMed]
- Zhang YS, Zhang YH, Li XJ, et al. Bystander effect and abscopal effect in recurrent thymic carcinoma treated with carbon-ion radiation therapy: A case report. World J Clin Cases 2021;9:6538-43. [Crossref] [PubMed]
- Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol 1953;26:234-41. [Crossref] [PubMed]
- Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004;58:862-70. [Crossref] [PubMed]
Cite this article as: Kishi N, Matsuo Y. Postoperative radiotherapy for thymic epithelial tumors: a narrative review. Mediastinum 2024;8:40.