
Perioperative strategies and management of giant anterior mediastinal tumors: a narrative review
Introduction
Background
Giant anterior mediastinal tumors may cause severe hemodynamic and respiratory decompensation due to mass effects (1-11). Since mediastinal space is narrow, giant anterior mediastinal tumors are susceptible to mechanical compression or infiltration of the surrounding organs (4). The circulatory collapse and respiratory failure related to the compression are known as mediastinal mass syndrome (MMS), which may occur during biopsy or surgery, even in the absence of any symptoms, owing to the supine position and the use of sedatives and muscle relaxants (1-8,10,11). MMS is a life-threatening condition that can occur not only during anesthesia but also at any time in patients with large mediastinal tumors (2,6,7,10). Therefore, multidisciplinary peri-operative management strategies are required.
Rationale and knowledge gap
No specific guidelines have been established for the management of patients undergoing surgery for giant anterior mediastinal masses. Anterior mediastinal tumors represent a diverse group of diseases, and their treatment varies depending on their diagnosis (5). A comprehensive preoperative assessment based on the differential diagnosis and risk evaluation for MMS is required.
Objective
This study aimed to summarize the evaluation methods for anterior giant mediastinal tumors and safe perioperative management strategies. We present this article in accordance with the Narrative Review reporting checklist (available at https://med.amegroups.com/article/view/10.21037/med-23-40/rc).
Methods
A literature review was conducted on August 19, 2023 by searching the PubMed database for the search terms (((giant) OR (huge)) AND (anterior) AND ((mediastinal) OR (mediastinum)) AND ((tumor) OR (mass))) and (mediastinal mass syndrome). Studies published over the past 10 years were selected for the analysis. Only studies published in English were included in this analysis. We also searched the references of the articles identified using this search strategy and selected those that were adjudged relevant. In this review, ((giant) OR (huge)) was defined as tumors >10 cm in diameter, with some selected from older references in the case of landmark papers or secondarily referenced in studies of interest (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | August 19th, 2023 |
| Databases and other sources searched | PubMed |
| Search terms used | (((giant) OR (huge)) AND (anterior) AND ((mediastinal) OR (mediastinum)) AND ((tumor) OR (mass))) |
| (mediastinal mass syndrome) | |
| Timeframe | 2013–2023 |
| Inclusion and exclusion criteria | Inclusion criteria: all types of articles available in full text in English [(giant) or (huge) are defined as a tumor larger than 10 cm in diameter, with some select older references in the case of landmark papers or if secondarily referenced in studies of interest] |
| Exclusion criteria: as for case reports, tumors less than 10 cm in diameter or not listed | |
| Selection process | The records were first screened for title or abstract by three independent reviewers (K.T., D.K. and T.M.), and subsequently screened for full text. Then, the articles were evaluated collectively by all authors. Debate over article selection was resolved with consensus |
Search findings
Giant anterior mediastinal tumors
A literature search of studies published over the past decade revealed that mature teratomas (12-29) were the most common large anterior mediastinal tumors, exceeding 10 cm in diameter. Some mature teratomas can grow rapidly and it has been estimated that approximately 15% of patients requires a resection extended to other structures (e.g., lobectomy, pericardiectomy) for complete tumor resection (4). Liposarcomas (30-40) were the second most common. Other tumors included thymomas (41-47), thymolipomas (48-52), thymic neuroendocrine tumors (NET) (53-56), malignant germ cell tumors (GCT) (57), and malignant lymphomas (58).
MMS
MMS is an acute hemodynamic and/or respiratory decompensation that occurs because of mechanical compression caused by mediastinal tumors (1-11). MMS can be aggravated by the induction of anesthesia or positional changes during surgery. However, it can occur at any stage of anesthesia (2,6,7,10). Asymptomatic patients can also develop MMS.
Hemodynamic decompensation occurs when an anterior mediastinal tumor compresses the heart and great vessels [superior vena cava (SVC) and pulmonary artery (PA)]. The induction of general anesthesia results in decreased venous return, which reduces right ventricular filling and causes low cardiac output (1,7). In the context of respiratory failure, supine positioning and positive pressure ventilation contribute to compression of the heart and great vessels, resulting in acute circulatory collapse (1,6,7). Compression of the SVC reduces the venous return of the upper half of the body (1,7). Therefore, edema of the face and upper extremities may occur (1,7,8). Furthermore, coughing, wheezing, dyspnea, and dysphagia are symptoms of pharyngeal and laryngeal edema (1). Cerebral edema causes headaches and disorders of consciousness. These conditions are referred to as SVC syndromes (1,6-8). PA compression may impair pulmonary perfusion, cause hypoxemia, acute right ventricular failure, and cardiac arrest.
Respiratory failure is caused by the mechanical compression of the trachea and/or main bronchus by a tumor. Induction of general anesthesia aggravates critical respiratory conditions (1,6,7,11). The use of sedatives or muscle relaxants causes a decrease in the respiratory muscle tone and elevation of the diaphragm (1,6,7,11). The position of the tumor changes, thereby increasing the risk of mechanical airway obstruction. Furthermore, loss of spontaneous breathing during controlled ventilation promotes the onset of respiratory complications (1,2,6,7). Positive pressure ventilation increases pleural pressure and strengthens the compression of mediastinal structures (1,6,7). The supine position during anesthesia induction also increases compression of the mediastinal structures because of the gravitational effect. This posture also causes a reduction in the transverse diameter of the thorax and cephalad displacement of the diaphragm, which increases the intrathoracic pressure, thereby promoting airway compression and impairing ventilation (7,59).
Tan et al. (2) reviewed 85 patients from 77 case reports. They reported that 48 (56.5%) patients had anterior mediastinal tumors with larger diameters than tumors of the superior, middle, and posterior mediastinum. MMS also occurred in 39 of 85 patients (45.9%), 25 of whom had anterior mediastinal tumors. Of the 39 patients who developed MMS, lymphomas were the most common, accounting for 15 (38.5%), suggesting that lymphomas may grow at a faster rate depending on the histologic type and that respiratory and circulatory compensations often do not occur in time.
Risk classification of MMS
Coordination of a multidisciplinary team (thoracic surgeons, anesthesiologists, respiratory medicine physicians, oncologists, radiologists, pathologists, cardiovascular surgeons, and clinical engineers) is indispensable for the management of giant anterior mediastinal tumors (3,5,7).
First, the presence of symptoms is identified. Preoperative orthopnea and upper body edema are high-risk factors for anesthesia-related complications in children (8). Orthopnea: P=0.033, odds ratio (OR) 5.31, 95% confidence interval (CI): 1.15–24.56, upper body edema: P=0.035, OR 8.00, 95% CI: 1.16–55.07 (8). Other symptoms included chest distress, dyspnea, swelling, tachycardia, cyanosis, and stridor (Table 2) (2,6). Symptomatic patients are generally at a higher risk of MMS (1-3,5-8,10). The prediction of perioperative complications were the occurrence of cardiorespiratory signs and symptoms at presentation (OR 6.2, 95% CI: 1.2–31.5) (10). It is also important to identify the ‘rescue position’, the position at which the symptoms are alleviated (1,2,5-8).
Table 2
| Risk classification | Signs and symptoms | Imaging examination findings |
|---|---|---|
| Safe | No | No |
| Unsafe | Yes: chest distress, dyspnea, swelling, tachycardia, cyanosis, orthopnea, stridor, SVC syndrome | Yes: tracheobronchial CSA <50% or compressed heart/vessel |
| Uncertain | Yes | No/NA |
| No | Yes |
SVC, superior vena cava; CSA, cross-sectional area; NA, not available.
Risk classification were based on symptoms, tracheobronchial compression, and heart/great vessel compression (Table 2) (1-3,5-7,10). Tracheal cross-sectional area (CSA) is an indicator of tracheal stenosis. A >50% reduction in normal CSA has been reported to increase the risk of perioperative respiratory complications (1-3,6-8,10) even in asymptomatic patients (3).
Differential diagnosis and preoperative management
Imaging study
Computed tomography (CT) can be used to confirm the location and size of a tumor and its relationship with the trachea, bronchi, heart, and great vessels (4-7). The evaluation of airway stenosis is also important. Contrast-enhanced CT allows for the differentiation of vascular abnormalities, evaluation of intratumoral necrosis, compression of the great vessels, and intravenous thrombus (1,60). Magnetic resonance imaging (MRI) is inferior to CT in terms of its spatial resolution but superior in qualitative evaluation. This provides valuable insight into the differential evaluation of solid and cystic lesions (60). MRI is a sensitive technique for differentiating soft tissues and delineating their boundaries. CT and MRI findings can predict the differential diagnoses (60). Solid masses included thymic epithelial tumors, thymic NETs, malignant lymphomas, GCTs, thymic hyperplasia, and thymolipomas. Cystic masses include thymic cysts, pericardial cysts, mature cystic teratomas, substernal goiters, ectopic parathyroid cysts, and neurogenic tumors (60). Positron emission tomography (PET)-CT is effective in differentiating benign and malignant thymic epithelial tumors because of differences in the maximum standardized uptake value (SUV) (61,62). However, it is not an appropriate tool for providing additional anatomical information (4).
Blood investigation data
Differential diagnoses can be made based on the blood investigation data. Patients positive for anti-acetylcholine receptor antibodies demonstrate an increased probability of thymoma even when they do not have myasthenia gravis (63). Levels of soluble interleukin-2 receptor (sIL-2R) are elevated in malignant lymphomas (63). If either alpha-fetoprotein (AFP) or beta-human chorionic gonadotropin (β-HCG) levels are elevated, the diagnosis of yolk sac tumor or choriocarcinoma, respectively, can be made, and a biopsy is not necessary (63,64). Malignant nonseminomatous germ cell tumor (NSGCT) can be diagnosed by abnormally high levels of AFP and β-HCG without waiting for biopsy or other pathology results, and chemotherapy can be initiated immediately in such cases (64).
Physiological function tests
Echocardiography can be used to evaluate the status of compression of the heart and great vessels, cardiac function, and the presence of pericardial effusion (2, 5, 10). Pulmonary function tests have been reported to have low sensitivity in predicting intraoperative respiratory complications (1). However, they can also be used to predict the risk of postoperative respiratory complications. Béchard et al. demonstrated that a peak expiratory flow rate (PEFR) of <40% was associated with a >10-fold increase in the risk of postoperative respiratory complications (P=0.010, OR =12.8, 95% CI: 1.5–47.1) (10). Bronchoscopy can also be used to evaluate the airway stenosis. The rescue position can be ascertained by changing the patient’s position (5).
Biopsy
Biopsy is of paramount importance because the treatment varies depending on the pathological diagnosis (Figure 1). Biopsy techniques include ultrasound-guided endoscopic biopsy, percutaneous image-guided needle biopsy, parasternal anterior mediastinotomy, cervical mediastinoscopy, video-assisted thoracoscopic surgery (VATS), and open surgery (4,63). However, for giant tumors, biopsy under general anesthesia increases the risk of MMS (9,11). If the MMS risk classification is ‘unsafe’ or ‘uncertain’, biopsy in a comfortable position with local anesthesia is preferred. Cytopathological evaluation of pleural effusions or biopsies of palpable lymph nodes on the body surface may provide a definitive diagnosis (6,65). Early initiation of chemotherapy after a definitive diagnosis is important to improve the survival of patients with malignant lymphoma (4,58). Among malignant GCT, seminomas respond to chemotherapy and radiotherapy (64). NSGCT are treated with chemotherapy, followed by surgical resection of residual tumors (57). If the imaging findings are those of a cystic lesion and there is no elevation in the levels of tumor markers, although cancer antigen-125 (CA-125) may be elevated in the presence of inflammation (20), a mature cystic teratoma is more likely to be diagnosed. Image-guided drainage results in tumor shrinkage reduces the risk of MMS (12,19,24,28). If thymoma is strongly suspected and resectable, a penetrating biopsy of the pleura should be avoided because of its potential for dissemination (66). Preoperative chemotherapy or radiotherapy is indicated for complete resection of locally advanced thymomas (4,41,43,44,46,67).

Intraoperative management
If the MMS risk classification is ‘unsafe’ or ‘uncertain’, intraoperative management deserves special attention as follows (2).
Rescue position
It is important to determine the patient’s comfortable position or ‘rescue position’. The Fowler’s position (elevated upper body) is reportedly effective in patients with SVC syndrome (7). Lateral decubitus (59) and prone (68) positions are also useful. A positional change in the contralateral direction in which the tumor weight is applied (e.g., left lateral decubitus position if the trachea is compressed mainly from the left thoracic cavity on CT images) is usually selected. However, if the patient is placed in a prone position, it is difficult to establish extracorporeal membrane oxygenation (ECMO). Leaving patients in ‘rescue position’ as long as possible during any induction for anesthesia is important.
Airway management
If the tracheal carina or main bronchus is compressed, the intubation tube is advanced beyond the stenosis to ventilate the distal airway. The double-lumen tube allows for intubation beyond the distal airway stenosis and provides good surgical vision during tumor resection by providing isolated lung ventilation (5). Intubation beyond the distal portion of the stenosis is expected to be difficult in malignant cases because the tumors are generally firm. In these cases, the use of a rigid bronchoscope should be considered. In cases with airway emergency, temporary endotracheal stenting is effective after establishment of veno-venous ECMO (VV-ECMO) under local anesthesia (58,69).
ECMO
Indications for ECMO are cardiac support, respiratory support or a combination of both (70). Veno-arterial ECMO (VA-ECMO) is preferred in patients at high risk of respiratory and hemodynamic collapse. VV-ECMO should be used in patients with isolated respiratory symptoms and airway compression (1). If respiratory and/or circulatory distress is obvious in adults, a sheath should be placed on the femoral artery and vein (depending on the type of ECMO setup chosen), so that ECMO can be introduced promptly before the induction of general anesthesia (1-3,5-7). Clinical engineers should be available in the operating room for the whole duration of surgery with a primed ECMO machine (1,7). Furthermore, ECMO should be on standby in asymptomatic patients with highly compressed trachea, main bronchus, heart, and great vessels. In infants, it is difficult to administer ECMO under local anesthesia. Ramanathan et al. (3) advocated that the following cases should be considered a ‘high-risk’ group: (I) acute SVC syndrome, (II) PA or right ventricular outflow tract (RVOT) obstruction, (III) > 50% airway compression, and (IV) cardiac or great vessel involvement/invasion with possible need for cardiac or vascular excision or reconstruction.
Induction of anesthesia
In cases of SVC compression or obstruction, intravenous access routes should be secured in the lower extremities (femoral vein) (1,5-7,10). The administered drug cannot be reliably perfused in SVC syndrome, and there is a risk of further increase in the central venous pressure. The use of short-acting medications is important to maintain normal muscle tone and adequate spontaneous breathing (1,2,5,7). Therefore, careful attention should be paid to the use of sedatives and muscle relaxants in these patients. Ideally, airway management should be performed while the patient is awake and maintains spontaneous breathing (7,56). Erdös et al. (7) delineated the following 12 rules for perioperative anesthesia management of MMS in adults: (I) interdisciplinary team consultation; (II) possibility of irradiation/chemotherapy; (III) clinical/radiological findings; (IV) MMS-risk classification; (V) availability of adequate number of staff; (VI) no premedication; (VII) transportation with anesthesiologist; (VIII) intravenous access in the lower extremity; (IX) pulse oximetry to the right arm; (X) artery catheter/central venous access; (XI) flexible operating table. ‘comfortable’ position; (XII) alternative airway/circulation options (e.g., cannulation of femoral vessels).
MMS occurrence
In the event of occurrence of MMS, the patient should be repositioned to the ‘rescue position’ by moving the operating table. During respiratory decompensation, the intubation tube should be advanced beyond the obstruction area to ventilate the distal airway section or a rigid bronchoscope should be inserted. Inotropes and hyperosmolar solutions must be administered to prevent circulatory collapse. If there is no improvement, ECMO should be established, and emergency thoracotomy should be performed quickly to release the mechanical compression of the tumor.
Figure 2 shows a flowchart of the perioperative management and strategies for giant anterior mediastinal tumors (2,3,5).
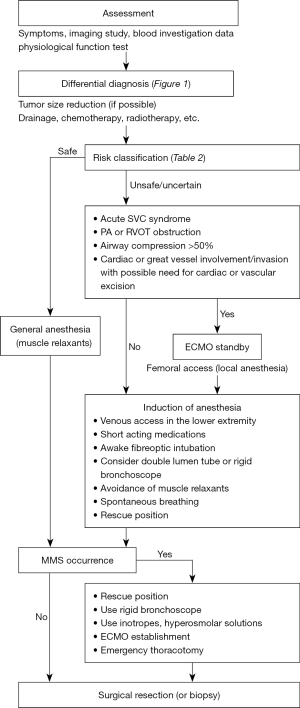
Surgical approach
A surgical approach is essential to reach and resect the tumor properly. Median sternotomy is a commonly used approach for mediastinal tumor resection (4). It allows better exposure of the superior and anterior mediastinal lesions. However, it is not suitable for tumors extending into the thoracic cavity because of poor access to the hilum or posterior thorax (47).
Lateral thoracotomy was considered when the tumor was unevenly distributed to the right or left. Lateral thoracotomy combined with median full sternotomy (20,25,27,39) and upper partial sternotomy combined with lateral thoracotomy [hemi-clamshell thoracotomy (47)] are effective for achieving good surgical vision.
Clamshell thoracotomy (13,30,32,37,38,40,44), although highly invasive, involves a large transverse incision that allows access to the bilateral pleural lumen. Sometimes, a longitudinal sternal incision is added to clamshell thoracotomy (40,44). The addition of an upper partial sternotomy provides a good view of the superior mediastinum, while the addition of a lower partial sternotomy is useful for the resection of tumors situated on the diaphragm.
VATS has also been used in some cases (16,26,29,45). However, this approach requires the use of advanced techniques. VATS may be effective in a limited number of giant anterior mediastinal tumors.
Postoperative management
Cases classified as ‘unsafe’ or ‘uncertain’ according to the risk classification should be managed postoperatively in the intensive care unit. Early extubation reduces the risk of postoperative complications. Therefore, adequate pain management is recommended. Epidural anesthesia was effective. However, it is difficult to use this technique in patients at a risk of hypotension or coagulopathy. Systemic heparinization is necessary in cases of ECMO and cardiopulmonary bypass; however, the risk of hematoma with epidural catheters remains controversial. An erector spinae plane block (1) or thoracic paravertebral block may be an effective alternative to epidural anesthesia.
Strengths and limitations
This article describes the differential diagnosis of giant anterior mediastinal tumors and the corresponding strategies based on the MMS risk classification. However, as mediastinal tumors are rare, this review does not encompass all entities.
Conclusions
When encountering a giant anterior mediastinal tumor, the degree of symptoms should be checked, and the status of the airway and heart/great vessel compression should be confirmed using imaging studies. Risk assessments of MMS and differential diagnoses should be performed simultaneously. Depending on the differential diagnosis, tumor reduction following surgery is effective. Biopsy and surgery must be carefully planned and multidisciplinary team coordination is essential.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://med.amegroups.com/article/view/10.21037/med-23-40/rc
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-23-40/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-23-40/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bertini P, Marabotti A. The anesthetic management and the role of extracorporeal membrane oxygenation for giant mediastinal tumor surgery. Mediastinum 2023;7:2. [Crossref] [PubMed]
- Tan JC, Lin PS, He LX, et al. Anesthetic management of patients undergoing mediastinal mass operation. Front Surg 2022;9:1033349. [Crossref] [PubMed]
- Ramanathan K, Leow L, Mithiran H. ECMO and adult mediastinal masses. Indian J Thorac Cardiovasc Surg 2021;37:338-43. [Crossref] [PubMed]
- Aprile V, Korasidis S, Bacchin D, et al. Extended surgery of antero-superior mediastinum. Curr Chall Thorac Surg 2019;1:21. [Crossref]
- Li WW, van Boven WJ, Annema JT, et al. Management of large mediastinal masses: surgical and anesthesiological considerations. J Thorac Dis 2016;8:E175-84. [Crossref] [PubMed]
- Blank RS, de Souza DG. Anesthetic management of patients with an anterior mediastinal mass: continuing professional development. Can J Anaesth 2011;58:853-9, 860-7. [Crossref] [PubMed]
- Erdös G, Tzanova I. Perioperative anaesthetic management of mediastinal mass in adults. Eur J Anaesthesiol 2009;26:627-32. [Crossref] [PubMed]
- Anghelescu DL, Burgoyne LL, Liu T, et al. Clinical and diagnostic imaging findings predict anesthetic complications in children presenting with malignant mediastinal masses. Paediatr Anaesth 2007;17:1090-8. [Crossref] [PubMed]
- Inoue M, Minami M, Shiono H, et al. Efficient clinical application of percutaneous cardiopulmonary support for perioperative management of a huge anterior mediastinal tumor. J Thorac Cardiovasc Surg 2006;131:755-6. [Crossref] [PubMed]
- Béchard P, Létourneau L, Lacasse Y, et al. Perioperative cardiorespiratory complications in adults with mediastinal mass: incidence and risk factors. Anesthesiology 2004;100:826-34; discussion 5A. [Crossref] [PubMed]
- Takeda S, Miyoshi S, Omori K, et al. Surgical rescue for life-threatening hypoxemia caused by a mediastinal tumor. Ann Thorac Surg 1999;68:2324-6. [Crossref] [PubMed]
- Tani K, Kimura D, Matsuo T, et al. Rescue computed tomography-guided drainage of a giant mediastinal mature teratoma causing airway obstruction prior to surgical resection: a case report. Surg Case Rep 2023;9:59. [Crossref] [PubMed]
- Bendre PS, Banerjee A, Munghate G, et al. Application of the Clamshell Thoracotomy in an Infant with a Large Mediastinal Tumor. J Indian Assoc Pediatr Surg 2023;28:170-2. [Crossref] [PubMed]
- Howell RS, Magid MS, Kuenzler KA, et al. Giant mediastinal teratoma in a young infant: a case report. Mediastinum 2022;6:25. [Crossref] [PubMed]
- Manabe T, Kajiyama K, Iwanami T, et al. Unusual giant multilocular thymic cyst with mature teratoma including a carcinoid component in the mediastinum. Surg Case Rep 2022;8:24. [Crossref] [PubMed]
- Zhang J, Zhu X, Jiang M, et al. A ruptured giant mediastinal mature teratoma mimicking an encapsulated empyema. Interact Cardiovasc Thorac Surg 2022;34:159-61. [Crossref] [PubMed]
- Piber N, Weichert W, Hörer J, et al. Life-Threatening Mediastinal Teratoma of Infant Requiring Emergency Surgical Removal. Thorac Cardiovasc Surg Rep 2022;11:e7-e10. [Crossref] [PubMed]
- Luo Q, Qu W, Chen C, et al. Compression of superior vena cava and azygos vein by a giant mediastinal mature cystic teratoma: a case report. Transl Cancer Res 2021;10:4577-81. [Crossref] [PubMed]
- Shiomi S, Mori S, Shigemori R, et al. Avoidance of circulatory collapse by preoperative percutaneous drainage of tumor contents for a giant mediastinal mature cystic teratoma. Gen Thorac Cardiovasc Surg 2021;69:401-4. [Crossref] [PubMed]
- Bellido-Yarlequé D, Meza K, Palacios J, et al. Giant benign teratoma occupying the left hemithorax with pleural effusion: a rare presentation. J Surg Case Rep 2020;2020:rjaa294. [Crossref] [PubMed]
- Ahmed SS, Samad K, Yousuf MS, et al. Paediatric Thoracic Tumour Resection: Challenge For An Anaesthesiologist. J Ayub Med Coll Abbottabad 2020;32:132-5. [PubMed]
- Ryan E, Shennib H, Gopal S. Giant intrathoracic teratoma presenting with cachexia and severe dyspnea. J Cardiothorac Surg 2019;14:96. [Crossref] [PubMed]
- Kumar A, Persuad P, Shiwalkar N. Intraoperative Catastrophe during Benign Mediastinal Tumor Mass Excision: A Case Report. Cureus 2019;11:e4941. [Crossref] [PubMed]
- Brenn BR, Reddy SK, Van Arendonk KJ, et al. Perioperative management of an anterior mediastinal teratoma in an infant: one more tool in the toolbox. BMJ Case Rep 2018;2018:bcr2018227022. [Crossref] [PubMed]
- Takanashi Y, Tajima S, Takahashi T, et al. Mediastinal mature teratoma with complete gastrointestinal and bronchial walls. Respirol Case Rep 2015;3:89-91. [Crossref] [PubMed]
- Miyauchi Y, Matsubara H, Uchida T, et al. Successful thoracoscopic removal of a giant teratoma following extraction of cystic conponents: a case report. Asian J Endosc Surg 2014;7:79-81. [Crossref] [PubMed]
- Yokoyama Y, Chen F, Date H. Surgical resection of a giant mediastinal teratoma occupying the entire left hemithorax. Gen Thorac Cardiovasc Surg 2014;62:255-7. [Crossref] [PubMed]
- Dalal U, Jora MS, Dalal AK, et al. Primary germ cell tumor of the mediastinum - presenting as a huge mass. Int J Prev Med 2014;5:230-2. [PubMed]
- Rothermel L, Gilkeson R, Markowitz AH, et al. Thoracoscopic resection of a giant teratoma compressing the right heart. Interact Cardiovasc Thorac Surg 2013;17:594-7. [Crossref] [PubMed]
- Al Kindi AH, Al Kindi FA, Al Riyami M, et al. Giant Mediastinal Myxoid Pleomorphic Liposarcoma. Sultan Qaboos Univ Med J 2023;23:271-3. [PubMed]
- Alhames S, Ghabally M. Enbloc resection of the largest thymic liposarcoma: A case report with literature review. Ann Med Surg (Lond) 2020;59:204-6. [Crossref] [PubMed]
- Gaikwad NM, Srikrishna SV, Srikanth K. A rare case of a giant anterior mediastinal liposarcoma. Indian J Thorac Cardiovasc Surg 2020;36:148-50. [Crossref] [PubMed]
- Zhang H, Yimin N, He Z, et al. Giant mediastinal liposarcoma resected by median sternotomy: a case report. Transl Cancer Res 2020;9:6522-7. [Crossref] [PubMed]
- Iwamoto N, Matsuura Y, Ninomiya H, et al. An extremely rare case of rapidly growing mediastinal well-differentiated liposarcoma with a sclerosing variant: a case report. Surg Case Rep 2020;6:158. [Crossref] [PubMed]
- Zhang M, Zhang S, Shi H, et al. Resection of a huge mediastinal well-differentiated liposarcoma involving left thoracic cavity. J Cardiothorac Surg 2019;14:148. [Crossref] [PubMed]
- Yang YS, Bai CY, Li ZC, et al. Giant primary liposarcoma of the anterior mediastinum: A case report. Medicine (Baltimore) 2018;97:e12873. [Crossref] [PubMed]
- Sugiura Y, Hashizume T, Fujimoto H, et al. A giant mediastinal liposarcoma weighing 3500g resected with clam shell approach, a case report with review of literature. Int J Surg Case Rep 2017;41:292-5. [Crossref] [PubMed]
- Toda M, Izumi N, Tsukioka T, et al. Huge mediastinal liposarcoma resected by clamshell thoracotomy: a case report. Surg Case Rep 2017;3:16. [Crossref] [PubMed]
- Huang W, Jiang GN. Resection of giant mediastinal liposarcoma via '⊣ shape' incision. J Surg Case Rep 2017;2017:rjw219. [Crossref] [PubMed]
- Hirano Y, Yamamoto H, Ichimura K, et al. Surgical resection of a massive primary mediastinal liposarcoma using clamshell incision combined with lower median sternotomy: report of a case. Ann Thorac Cardiovasc Surg 2014;20:606-8. [Crossref] [PubMed]
- Policarpo F, Antunes M, Alvoeiro M, et al. Incidental giant thymoma-a reminder of the importance of a global look of the imaging scans. J Surg Case Rep 2023;2023:rjad084. [Crossref] [PubMed]
- Wang R, Yang X, Zhu W, et al. Huge thymoma combined with pure red cell aplasia: a case report and literature review. Gland Surg 2022;11:938-42. [Crossref] [PubMed]
- Çap M, Erdoğan E, Akyüz A, et al. Progressive pulmonary stenosis due to huge mediastinal thymoma. Anatol J Cardiol 2021;25:E28-9. [Crossref] [PubMed]
- Lampridis S, Bilkhu R, Lucchese G, et al. Complete Surgical Resection of a Giant Invasive Thymoma with Right Pneumonectomy and Graft Reconstruction of the Superior Vena Cava and Left Brachiocephalic Vein: A Case Report. Case Rep Surg 2022;2022:9604926. [Crossref] [PubMed]
- Gonzalez-Rivas D, Wu CF, de la Torre M. Uniportal video-assisted thoracoscopic thymectomy and resection of a giant thymoma in a patient witness of Jehova. J Thorac Dis 2017;9:E556-9. [Crossref] [PubMed]
- Kojima H, Isaka M, Nagata M, et al. Preoperative Proton Beam Therapy for Thymoma: A Case Report. Ann Thorac Cardiovasc Surg 2016;22:186-8. [Crossref] [PubMed]
- Zhao W, Fang W. Giant thymoma successfully resected via hemiclamshell thoracotomy: a case report. J Thorac Dis 2016;8:E677-80. [Crossref] [PubMed]
- Joseph HT, Rathi RK, Goel H, et al. Symptomatic giant thymolipoma in a child. Lung India 2023;40:275-8. [Crossref] [PubMed]
- Gong LH, Wang WX, Zhou Y, et al. Surgical resection of a giant thymolipoma causing respiratory failure: A case report. World J Clin Cases 2023;11:1137-43. [Crossref] [PubMed]
- Otido S, Dangor Z, Zanini A, et al. Giant thymolipoma in a child: The silent chest mass. Afr J Thorac Crit Care Med 2022; [Crossref] [PubMed]
- Wang H, Li Q, Jiang Q, et al. A giant tumor of the mediastinum mixed with thymocytes and adipocytes: a case report. Gland Surg 2021;10:3163-6. [Crossref] [PubMed]
- Aghajanzadeh M, Asgary MR, Mesbah A, et al. Giant thymolipoma of mediastinum and neck - initially misdiagnosed as liposarcoma by core needle biopsy. J Family Med Prim Care 2018;7:1079-82. [Crossref] [PubMed]
- Gupta K, Patel P, Patibandla S, et al. Rare Acute Presentation of a Low-Grade Thymic Neuroendocrine Tumor. Cureus 2020;12:e10850. [Crossref] [PubMed]
- Jun JE, Hwang YC, Ahn KJ, et al. A rare case of multiple endocrine neoplasia type 1 initially presenting as an asymptomatic, huge mediastinal mass: case report. BMC Endocr Disord 2021;21:31. [Crossref] [PubMed]
- Rumbinaitė E, Dirsienė R, Vaitiekus D, et al. Is this dyspnea because of coronary artery disease? A rare case report of primary neuroendocrine tumor in anterior mediastinum. Perfusion 2023; Epub ahead of print. [Crossref] [PubMed]
- Kawagoe I, Satoh D, Mitaka C, et al. Specific airway management for SVC replacement during giant anterior mediastinal tumor resection. JA Clin Rep 2020;6:70. [Crossref] [PubMed]
- Chaudhry IU, Rahhal M, Khurshid I, et al. Radical surgical resection for giant primary mediastinal endodermal sinus tumour with pulmonary metastasis after chemotherapy: can be curative. BMJ Case Rep 2014;2014:bcr2014204662. [Crossref] [PubMed]
- Oyake M, Suenobu S, Miyawaki M, et al. Airway Emergencies Due to Anterior Mediastinal T-Lymphoblastic Lymphoma Managed With Planned Extracorporeal Membrane Oxygenation and Endotracheal Stent: A Case Report and Literature Review. Cureus 2022;14:e21799. [Crossref] [PubMed]
- Choi WJ, Kim YH, Mok JM, et al. Patient repositioning and the amelioration of airway obstruction by an anterior mediastinal tumor during general anesthesia -A case report-. Korean J Anesthesiol 2010;59:206-9. [Crossref] [PubMed]
- Nakazono T, Yamaguchi K, Egashira R, et al. Anterior mediastinal lesions: CT and MRI features and differential diagnosis. Jpn J Radiol 2021;39:101-17. [Crossref] [PubMed]
- Fukumoto K, Taniguchi T, Ishikawa Y, et al. The utility of [18F]-fluorodeoxyglucose positron emission tomography-computed tomography in thymic epithelial tumours. Eur J Cardiothorac Surg 2012;42:e152-6. [Crossref] [PubMed]
- Treglia G, Sadeghi R, Giovanella L, et al. Is (18)F-FDG PET useful in predicting the WHO grade of malignancy in thymic epithelial tumors? A meta-analysis. Lung Cancer 2014;86:5-13. [Crossref] [PubMed]
- Date H. Diagnostic strategies for mediastinal tumors and cysts. Thorac Surg Clin 2009;19:29-35. vi. [Crossref] [PubMed]
- Pini GM, Colecchia M. Mediastinal germ cell tumors: a narrative review of their traits and aggressiveness features. Mediastinum 2022;6:5. [Crossref] [PubMed]
- Malik R, Mullassery D, Kleine-Brueggeney M, et al. Anterior mediastinal masses - A multidisciplinary pathway for safe diagnostic procedures. J Pediatr Surg 2019;54:251-4. [Crossref] [PubMed]
- Kattach H, Hasan S, Clelland C, et al. Seeding of stage I thymoma into the chest wall 12 years after needle biopsy. Ann Thorac Surg 2005;79:323-4. [Crossref] [PubMed]
- Onuki T, Ishikawa S, Yamamoto T, et al. Pathologic radioresponse of preoperatively irradiated invasive thymomas. J Thorac Oncol 2008;3:270-6. [Crossref] [PubMed]
- Armas A, Primm AN. Anesthetic Management of a Patient With an Anterior Mediastinal Mass Undergoing Endoscopic Retrograde Cholangiopancreatography in the Prone Position: A Case Report. A A Pract 2020;14:25-7. [Crossref] [PubMed]
- Matsumoto R, Mitsuoka M, Hashiguchi T, et al. Temporary airway stenting for giant anterior mediastinal tumor biopsy: Two case reports. Int J Surg Case Rep 2019;64:157-60. [Crossref] [PubMed]
- Aprile V, Korasidis S, Ambrogi MC, et al. Extracorporeal membrane oxygenation in traumatic tracheal injuries: a bold life-saving option. J Thorac Dis 2019;11:2660-3. [Crossref] [PubMed]
Cite this article as: Tani K, Kimura D, Matsuo T, Sasaki T, Kimura S, Muto C, Minakawa M. Perioperative strategies and management of giant anterior mediastinal tumors: a narrative review. Mediastinum 2024;8:34.


