
China Anti-Cancer Association Guidelines for the diagnosis, treatment, and follow-up of thymic epithelial tumors (2023)
Epidemiology
Common types of mediastinal lesions
Mediastinal lesions may be tumors [e.g., thymoma (TM), thymic carcinoma (TC), neuroendocrine thymic tumor (NETT), malignant lymphomas, germ cell tumors, thymic lipoma, extra-thoracic metastatic tumor] or non-neoplastic diseases (e.g., intrathoracic goiter, thymic cyst, and aortic aneurysm). Many mediastinal lesions are benign, especially those found in asymptomatic patients. On the other hand, patients with symptoms usually have malignant mediastinal lesions. All patients with mediastinal lesions should be evaluated to determine the possible histological type and extent of invasion prior to treatment. Before treatment, it is important to differentiate thymic epithelial tumors (TETs) from other diseases (e.g., lung metastases, lymphomas, goiter, and germ cell tumors) because these diseases are treated differently. Most metastatic mediastinal masses are from primary lung cancer (e.g., non-small cell lung cancer). However, approximately 50% of primary tumors in the anterior mediastinum are TETs in adults.
TET, especially TM, usually develops slowly, while the symptoms of lymphoma or malignant germ cell tumor develop rapidly. Lymphomas typically present as a systemic disease but may also present as primary anterior mediastinal lesions [e.g., nodular sclerosis Hodgkin’s lymphoma and non-Hodgkin’s lymphoma (e.g., primary mediastinal large B-cell lymphoma and T lymphoblastic lymphoma)]. Patients usually have lymphadenopathy accompanied by elevated serum lactate dehydrogenase (LDH). However, thymic extranodal marginal zone lymphoma of mucosal-associated lymphoid tissue (MALT lymphoma) is a type of clinically indolent non-Hodgkin lymphoma. Systemic “B symptoms” (i.e., fevers, night sweats, weight loss) are uncommon (1). Many patients with MALT lymphoma have a history of autoimmune disease (e.g., Sjögren’s syndrome, systemic lupus erythematosus, or relapsing polychondritis, Hashimoto’s thyroiditis) (2). Extragonadal germ cell tumors are rare and may also occur in the mediastinum.
Epidemiological characteristics of TETs
TETs originate from the thymus, including TMs, TCs and NETTs.
TETs were previously considered a rare type of tumors, with an incidence of 0.30/100,000 depending on the Surveillance, Epidemiology, and End Results (SEER) database (3). However, in recent years, with the popularization of chest computed tomography (CT) screening for lung cancer, an increasing number of TETs have been detected in physical examinations, and the incidence may exceed 100 times higher than previously thought (4-6).
TMs usually occur in patients between 40 and 70 years old and rarely in children or teenagers. The etiology of TMs is unknown. Alcohol, smoking and ionizing radiation do not seem to be risk factors for TMs. The higher incidence of TMs among African Americans and Asia-Pacific islanders suggests a possible genetic factor. Some patients have no symptoms, but others may have chest pain, cough, dyspnea or superior vena cava syndrome. Approximately 30–50% of patients with TMs are complicated with myasthenia gravis, followed by pure red blood cell aplastic anemia, hypogammaglobulinemia, dermatomyositis, etc. The symptoms suggestive of myasthenia gravis include ptosis, diplopia, salivation, difficulty climbing upstairs, hoarseness and/or dyspnea. For all patients suspected of myasthenia gravis, it is recommended to measure the level of serum anti-acetylcholine receptor antibodies to determine the occurrence of myasthenia gravis to avoid respiratory failure during the perioperative period. If myasthenia gravis is combined, preoperative evaluation and treatment by a neurologist are recommended.
TCs are rare, aggressive tumors that have higher risk of metastasis to regional lymph nodes and extra-thoracic locations than TMs; thus, the prognosis of TCs is worse than that of TMs. The survival rate of TCs varies by the stage (stage I–II: 91%; stage III–IV: 31%) and resectability (7). TCs can be differentiated from TMs due to different histological morphology, immunohistochemical and genetic characteristics. However, TCs should be differentiated from metastases of extrathymic tumors. They have similar histological features, but some immunohistochemical markers can be used for differential diagnosis.
Notably, the clinical course of TCs is different from that of TMs. Paraneoplastic syndromes (including myasthenia gravis) are very rare in patients with TCs. If the diagnosis of myasthenia gravis is established, the pathological diagnosis of TC should be reassessed. The patient may actually have a TM.
NETT, with an incidence of 0.18/1,000,000 (8), is a subtype of TET that is rarer than TM and TC, accounting for 2–5% of all TETs. According to the SEER database, the average age of patients with NETTs is 55 years old, which is more common in men (8). It was reported that approximately 25% of patients with thymic carcinoid tumors have a family history of type I multiple neuroendocrine tumors (MEN1), and 17% to 30% of adults have paraneoplastic syndromes (e.g., Cushing syndrome). NETTs have a higher degree of malignancy and are more likely to have lymph node and distant metastasis than TCs.
Prevention and screening of mediastinal lesions
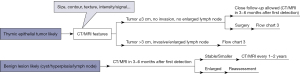
Recommendation (1B): CT screening for TETs is not recommended at present. Recommendation (1B): For small anterior mediastinal nodules detected on physical examination or by accident, magnetic resonance imaging (MRI) is recommended for differential diagnosis (Figure 1). If benign lesions (thymic cyst, thymic hyperplasia, small lymph node) are considered, follow-up by CT or MRI is recommended in 3–6 months and then every 1–2 years. Nontherapeutic surgery should be avoided for asymptomatic benign lesions. In patients with small anterior mediastinal lesions ≤3 cm, upfront surgery is recommended when high-grade TETs (type B2/B3 TM, TC, NETT) are suspected, but when low-grade TMs (type A/AB/B1) are suspected, either upfront surgery or close follow-up can be applied (Figure 2).

At present, there is no data available for the prevention of mediastinal lesions.
There is no evidence suggesting that low-dose CT screening can improve the prognosis of patients with TMs and TCs. Considering the low incidence of TETs, low-dose CT screening for TETs is not recommended at present. However, for patients diagnosed with autoimmune diseases (e.g., myasthenia gravis) and MEN1 diseases (9), targeted screening for TETs should be performed with chest CT.
The current Guidelines from National Comprehensive Cancer Network (NCCN) or European Society for Medical Oncology (ESMO) do not have relative recommendations for managing accidentally detected asymptomatic anterior mediastinal nodules (usually considered to be ≤3 cm in diameter). According to a study of 419 patients with asymptomatic small anterior mediastinal nodules (10), the majority of these lesions (65.6%) were benign cysts that remained stable during follow-up. Incorporating MRI with CT scans is helpful for differential diagnosis. Follow-up is suitable and safe for benign lesions, such as thymic cysts, thymic hyperplasia, and small lymph nodes. High-grade TETs (type B2/B3 TM, TC, NETT) need upfront surgery. In low-grade TMs (type A/AB/B1), these small nodules are usually well-demarcated, and the median tumor doubling time can be longer than one year. Thus, repeating CT/MRI 6 months after the first detection seems safe in patients with low-grade lesions.
Diagnosis and staging
Clinical differential diagnosis of mediastinal lesions
Recommendation (1B): The examinations for the clinical differential diagnosis of mediastinal lesions include blood biochemical tests, enhanced chest CT and MRI (Figure 1). Enhanced chest MRI is recommended to differentiate between mediastinal cystic and solid lesions, cystic and necrotic areas in solid lesions, septa and soft-tissue areas in cystic lesions, and TM and thymic hyperplasia/insufficient degeneration. Positron emission tomography/computed tomography (PET/CT) is used to detect the existence of recurrence or metastasis for invasive or aggressive tumors, as well as to assist in clinical staging and assess the therapeutic effect. Octreotide scan is preferable for highly suspected NETTs and screen for the potential treatment of somatostatin analogs (SSAs) for patients with NETTs.
Blood biochemical test
It has been reported that there is a low rate of elevated tumor markers in TETs. However, elevated serum cytokeratin 19 fragment (Cyfra 21-1) before surgery may suggest tumors in an advanced stage or with more aggressive behavior (11), thus indicating a higher risk of recurrence after resection. In addition, elevation of serum CA125 may be related to pleural effusion (11). Negative alpha-fetoprotein (AFP) and β-human chorionic gonadotropin (β-HCG) can generally exclude malignant germ cell tumors. Significant elevation of LDH indicates the possibility of lymphoma (12). A positive T-SPOT test indicates the possibility of mediastinal tuberculosis. Significant elevation of C-reaction protein (CRP) and erythrocyte sedimentation rate (ESR) indicates the possibility of mediastinal infection. Significant elevation of angiotensin-converting enzyme (ACE) indicates the possibility of sarcoidosis. For patients with suspected autoimmune diseases, specific antibodies should be tested. For patients with suspected paraneoplastic syndrome, hormonal workup should be done and guided by the presence of symptoms of the excess hormone (9).
Chest X-ray
In adults, the thymus is usually invisible on chest radiographs. Only when the size of a TET is large can it be detected on anteroposterior chest radiography, which generally shows a mass projecting to one side of the mediastinum. The tumor may also obscure the left or right heart border, and calcification in the tumor can also be seen on chest radiographs. On lateral radiographs, the tumor can present as an opacity posterior to the sternum and anterior to the aortic arch, main pulmonary artery, and heart. Other signs can also indicate the extent of tumor invasion, e.g., diaphragm elevation, pleural effusion, and pleural thickening. In general, chest radiographs are of limited value in the differential diagnosis and clinical staging of mediastinal lesions, and more effective imaging studies should be recommended.
Enhanced chest CT
The following features of mediastinal lesions should be considered on enhanced chest CT: localization; size; contours; texture (cystic, solid, or part-solid); density (with or without cystic change, necrosis, calcification, fatty tissue, and bleeding); the degree of enhancement (if any); the relationship between the mass and neighboring structures (invasion or not); with or without enlargement of mediastinal lymph nodes; with or without the occurrence of metastasis to the pleura, lung, bones, etc.
On CT scans, a TM usually presents as a round or oval mass with a clear boundary in the thymus, without lymphadenopathy. Aggressive TETs, such as TCs or NETTs, should be suspected when heterogeneous anterior mediastinal tumors are accompanied by local invasion, lymphadenopathy, and pleural effusion.
A lymphoma usually appears as a slightly enhanced soft tissue mass on CT, which usually surrounds and may even invade vessels. There might be enlargement of internal mammary lymph nodes that fuse with the tumor and enlargement of lymph nodes in the mediastinum, neck, axilla or other areas. In addition, when the combination of the above imaging features and typical “B” symptoms takes place in a young patient, a biopsy can usually confirm the diagnosis of lymphoma.
Retrosternal goiters and teratomas can be easily diagnosed using CT.
Among malignant germ cell tumors, seminomas are more common in young men. They present as homogeneously enhanced soft tissue masses on CT, although areas of cystic change or necrosis can be seen. Mixed germ cell tumors are heterogeneously enhanced masses with ill-defined areas of low attenuation secondary to necrosis, hemorrhage, or cystic change. Hematogenous metastasis is more likely to occur in these tumors.
Shen et al. studied the usefulness of CT features in accurate tumor staging before surgery (13). The results showed that the clinical staging of TET could be accurately evaluated with CT features, including tumor shape, contour, enhancement pattern, with or without invasion of adjacent structures, and presence of pleural or pericardial effusion or intrapulmonary metastasis.
However, there are limitations in the differential diagnosis of anterior mediastinal lesions by CT scan. This is mainly due to the difficulty in discriminating between benign cysts and tumors with cystic areas (e.g., cystic TM and MALT lymphoma). On CT scans, benign cysts are usually homogeneous, round or oval lesions with clear and smooth borders and water attenuation. In circumstances where cysts have high attenuation or appear as multilocular cystic lesions or with inflamed and thickened cystic walls, diagnosis by CT alone is difficult, and chest MRI is needed for further differentiation.
Enhanced chest MRI
For masses with high attenuation on CT scan, MRI is superior to CT in differentiating between mediastinal cystic and solid lesions, cystic and necrotic areas in solid lesions, and septa and soft tissue areas in cystic lesions (14). Dynamic contrast-enhanced images and curves can well detect cysts with high attenuation on CT as well as cysts with high signal intensity on T1-weighted images. An irregularly thickened and enhanced cyst wall is useful for differentiating cystic TETs from benign cysts. In addition, it is helpful for choosing a biopsy area when solid areas and cystic or necrotic areas inside the tumors can be demonstrated clearly.
The change in the signal intensity on dynamic contrast-enhanced images and curves can accurately evaluate the change in tumor cell viability after adjuvant/neoadjuvant therapy, which is superior to CT scan.
Chemical shift imaging of MRI can detect microscopic fatty tissue inside lesions through the phenomenon of reduced signal intensity in the opposed phase, which will not occur in TMs. Thus, it can be used to differentiate TMs from thymic hyperplasia or insufficient degeneration. In addition, this phenomenon does not occur in lymphomas.
On T2-weighted images, great vessels present low signal intensity due to the flow void effect, while mediastinal fat presents high signal intensity. Combined with enhanced MRI and CT, it is helpful to evaluate whether the tumor has invaded the vascular wall.
PET/CT
PET/CT is helpful in detecting the occurrence of metastasis to lymph nodes, lungs, pleura, or distant areas, but it is not recommended as a routine examination for thymic tumors. It can be used to evaluate the clinical staging of aggressive or advanced tumors and identify suspected recurrence and metastasis. PET/CT can also be used to evaluate tumor response to radiotherapy, chemotherapy, or other treatments.
Octreotide scan
Octreotide scans may help in the differential diagnosis of patients with highly suspected NETTs. In addition, for patients diagnosed with NETT, it can be used to evaluate whether they are suitable for the treatment of SSAs.
Pathological diagnosis of TET
The present guidelines follow the recommendations of the World Health Organization (WHO) Classification for tumors of the thymus (15). The main points of diagnosis are as follows.
TM
TMs are mainly classified into type A (including an atypical variant), AB, and B (separated into B1, B2 and B3) TMs by histology and immunohistochemistry (the morphology and atypia of neoplastic epithelial cells, the percentage of immature T cells, and so on).
Type A TMs usually contain mild spindle or oval tumor cells with few or no immature lymphocytes. In recent years, the concept of atypical type A TM has been proposed, characterized by a certain degree of atypical features, including hypercellularity, increased mitosis, and focal necrosis. Clinically they sometimes present with pulmonary metastases, which is extremely rare in typical type A TMs. However, due to the rarity of atypical type A TMs, their prognosis remains to be explored.
Type AB TMs usually consist of a component dominated by spindle cells (type A-like) with few lymphocytes and a lymphocyte-rich (type B-like) component. The ratio of the two components varies greatly among different patients.
Type B1 TMs are histologically similar to the normal thymus, where epithelial cells are scattered on the background of massive immature lymphocytes. The neoplastic epithelial cells are similar to the cortical epithelial cells. Medullary differentiation areas always exist.
Type B2 TMs consist of polygonal neoplastic epithelial cells intermingled with abundant immature T cells. Neoplastic epithelial cells are usually clustered, and the density of these cells is higher than that in type B1 TMs or normal thymus. Medullary differentiation areas could exist or not.
Type B3 TMs are mainly composed of mild to moderate atypical polygonal neoplastic epithelial cells arranged in sheets, with scarcity or absence of immature T cells.
Immunohistochemistry showed that immature lymphocytes express TDT, CD1a, and CD99, while neoplastic epithelial cells could express epithelial markers such as CK, CK19, and P63 but not CK20.
In addition, there are three rare types of TMs. Micronodular TM with lymphoid stroma is composed of multifocal, mild, spindle or oval cells surrounded by lymphoid stroma. A metaplastic TM is a biphasic tumor composed of solid areas of epithelial cells in a background of mild spindle cells. Lipofibroadenomas are similar to fibroadenomas of the breast.
TC and NETT
The diagnostic criteria of TC and NETT are similar to those of the corresponding tumors in other parts of the human body.
The most common type of TC is thymic squamous cell carcinoma. Positive CD5 and/or CD117 by immunohistochemistry usually indicate that squamous cell carcinoma originates from the thymus.
Micronodular TC with lymphoid hyperplasia is a new subtype of thymic squamous cell carcinoma with ‘non-organotypic’ lymphoid stroma that otherwise mimics micronodular TM with lymphoid stroma.
Lymphoepithelioma-like carcinomas, which resemble nasopharyngeal carcinomas in histology, are considered to be undifferentiated or poorly differentiated, with significant infiltration of lymphocytes and plasmocytes. Tumor cells are positive for Epstein-Barr virus in a significant number of cases.
Primary thymic adenocarcinomas are rare. Invasion or metastasis from adenocarcinomas elsewhere should be excluded before diagnosis.
A nuclear protein of the testis (NUT) carcinoma is a poorly differentiated tumor characterized by rearrangement of the NUT gene.
Undifferentiated carcinoma is an exclusionary diagnosis. Its histology and immunohistochemistry do not show the specific characteristics of TCs.
Basaloid carcinoma, mucoepidermoid carcinoma, clear cell carcinoma, sarcomatoid carcinoma, adenosquamous carcinoma, and TC NOS (not otherwise specific) occasionally occur.
The diagnostic criteria of subtypes of NETTs are similar to those of the lung. Differential diagnosis is generally not difficult.
Biopsy
Recommendation (1A): Biopsy is not recommended for a possible TET that can be resected upfront. Core-needle biopsy is recommended for tumors that cannot be resected upfront or might need non-surgical treatment. In circumstances where a core-needle biopsy cannot be performed (e.g., blocked by the sternum or lung tissue), biopsy via surgery, endobronchial ultrasonography (E-BUS), or mediastinoscope is suitable. However, in order to avoid tumor seeding into the pleural cavity which would impact the prognosis, biopsy via a trans-pleural approach is not routinely recommended for tumors without pleural metastasis.
For the pathological diagnosis of TETs using biopsy acquired samples, it is recommended to rule out the possibility of germ cell tumors and lymphomas first because they are also common in the mediastinum. The next step is to differentiate between NETTs and TMs or TCs. Last, it is best to differentiate between TMs and TCs. However, this may not always be feasible due to the limited volume of samples and the complicated histologic features. Further subtyping of a TM is also encouraged. For the purpose of accurate diagnosis, patients’ clinical information, including but not limited to gender, age, imaging features, tumor markers, and other relative test results, is also important.
Pathological report
Key points that should be demonstrated in a pathological report of surgical specimens are as follows. Further details can be found in the Thymic Epithelial Tumors Histopathology Reporting Guide (3rd edition) developed by International Collaboration on Cancer Reporting (ICCR) (16).
- Specimens submitted, including but not limited to the partial or total thymus, the primary tumor, co-resected tissues, lymph nodes, and/or metastatic lesions;
- Macroscopic findings of the specimens including the size, color, texture, with or without a capsule;
- Microscopic findings including the pathological subtype, invaded structures, status of resection margins, lymph node involvement, and pathological reaction to previous treatment;
- Results of immunohistochemical markers used for differential diagnosis.
Clinicopathologic staging of TETs
For decades, the Masaoka-Koga staging system (17) has been the most widely accepted staging system for thymic malignancies. In recent years, a new stage classification was proposed by the International Thymic Malignancies Interest Group (ITMIG) and the International Association for the Study of Lung Cancer (IASLC), which then formed the Union for International Cancer Control (UICC) TNM staging system (8th edition) for thymic malignancies (18). The current Chinese Anti-Cancer Association (CACA) guidelines recommend using the TNM staging system. Combined usage of the Masaoka-Koga staging system is allowed.
Treatment
Surgical treatment
The optimal treatment strategy for a TET patient should be proposed by a multidisciplinary team consisting of at least a thoracic surgeon, a radiologist, a pathologist, a medical oncologist, and a radiation oncologist. Whether a tumor can be resected completely is critical and should be evaluated by thoracic surgeons with experience in this field. The main principles of surgery are demonstrated in Table 1.
Table 1
| Principles of surgery |
| (I) Surgical resection should be performed after evaluation of the possibility of complete resection by thoracic surgeons and radiologists. Locally advanced tumors require cooperative treatment by a multidisciplinary team |
| (II) When a resectable thymic tumor is highly suspected based on clinical and radiological characteristics, surgical biopsy should be avoided considering the chance of iatrogenic pleural dissemination |
| (III) Before surgery, the signs and symptoms of myasthenia gravis should be evaluated and medically controlled |
| (IV) Goal of surgery is complete excision of the lesion with total thymectomy and complete resection of invaded tissues. Complete resection may require the removal of neighboring tissues, including the pericardium, phrenic nerve, pleura, lung, and even great vessels. Bilateral phrenic nerve resection should be avoided due to severe respiratory complications |
| (V) Surgical clips can be placed in areas with suspicious margins or residual lesions to help guide accurate postoperative radiotherapy |
| (VI) During surgery, exploration for the existence of pleural metastases should be performed. If feasible, resection of pleural metastases is recommended to achieve gross complete resection |
| (VII) Minimally invasive surgery is recommended for early-stage (UICC stage I or Masaoka-Koga stage I–II) tumors. In large clinical centers with mature techniques, minimally invasive surgery could be applied to UICC stage II–IIIa tumors |
| (VIII) Dissection of anterior mediastinal (N1) lymph nodes is recommended as a routine procedure, as N1 nodes are within the scope of total thymectomy. For tumors staged in T3 and above or those with high-grade histology (highly suspected or biopsy confirmed type B3 TM, TC, and NETT), further sampling of at least ipsilateral N2 lymph nodes is recommended. Bilateral N2 lymph node dissection is unnecessary, except for highly suspected NETTs with notable bilateral N2 nodes enlargement |
| Principles of radiotherapy |
| (I) A CT-based treatment plan prior to radiotherapy is highly recommended. Timely communication with the surgeon is helpful to determine the target volume |
| (II) For postoperative radiotherapy, the recommended radiation dose is 45–50 Gy for clean or close margins and 54 Gy for microscopically positive margins. For patients with unresectable tumors or with gross residual lesions, the recommended dose is 60–70 Gy (1.8–2 Gy/fx) |
| (III) The clinical target volume for postoperative radiotherapy should include the entire thymus, surgical clips, and potential residual lesions. The planning target volume should consider target motion and daily setup errors |
| (IV) At least a three-dimensional conformal technique should be adopted in radiotherapy to minimize the damage to surrounding normal tissues (e.g., heart, lung, esophagus, spinal cord). IMRT can further improve the dose distribution and reduce the irradiation dose of normal tissues. Proton therapy has better dosimetry thus favorable local control and toxicity than IMRT, and can be used in appropriate situations |
| (V) Given that patients with thymic tumors are relatively young and have long-term survival, it is recommended to minimize the dose volumes to normal tissues |
| Commonly used chemotherapy regimens |
| (I) CAP regimen: cisplatin 50 mg/m2 IV d1; doxorubicin 50 mg/m2 IV d1; cyclophosphamide 500 mg/m2 IV d1, every 3 weeks |
| (II) TC regimen: carboplatin AUC 6; paclitaxel 200 mg/m2, every 3 weeks |
| (III) PE regimen: cisplatin 60 mg/m2 IV d1; etoposide 120 mg/m2/day IV d1–3, every three weeks |
| (IV) ADOC regimen: cisplatin 50 mg/m2 IV d1; doxorubicin 40 mg/m2 IV d1; vincristine 0.6 mg/m2 IV d3; cyclophosphamide 700 mg/m2 IV d4, every 3 weeks |
| (V) Etoposide/ifosfamide/cisplatin regimen: etoposide 75 mg/m2 d1–4; ifosfamide 1.2 g/m2 d1–4; cisplatin 20 mg/m2 d1–4, every 3 weeks |
UICC, Union for International Cancer Control; TM, thymoma; TC, thymic carcinoma; NETT, neuroendocrine thymic tumor; CT, computed tomography; IMRT, intensity-modulated radiation therapy; AUC, area under the curve.
Surgical indication
Recommendation (Figure 3):
- Upfront surgery is recommended for resectable TETs (1A).
- For locally advanced tumors (staged in T3–4), re-evaluation for surgery after neoadjuvant therapy is recommended (1B).
- For patients with pleural dissemination or limited intrapulmonary metastases, upfront resection of the primary tumor and metastatic lesions or surgery after neoadjuvant therapy is recommended, depending on the resectability of the primary tumors (2C).
- For patients with mediastinal or pleural recurrence, surgical resection is an option and should be determined by a multidisciplinary team (1C).

Completeness of resection is one of the most important prognostic factors for TETs (19,20). For patients who can tolerate it, surgery is recommended for all resectable TETs. Therefore, it is very important to accurately assess the extent of tumor invasion before surgery. Shen et al. retrospectively analyzed the correlation between CT features and clinical staging or resectability among 138 TET patients receiving surgery (13). The results showed that the clinical staging of TETs could be evaluated via CT features, including tumor shape, contour, enhancement pattern, with or without invasion of adjacent structures, pleural or pericardial effusion, and intrapulmonary metastases. The absence of invasion of the great arteries on CT suggests the possibility of complete resection of a TET. Furthermore, chest MRI is more helpful in determining whether a tumor has invaded the vascular wall.
Common sites of recurrence for TETs, especially TMs, are the pleural cavity and primary tumor bed. A retrospective study based on the Japanese Association of the Research on the Thymus (JART) database analyzed the clinical characteristics of 405 patients with recurrent TETs (21). The results showed that 56.3% were in Masaoka stage I–III, and 25.9% were in stage IVa, suggesting that most recurrent tumors could still be resected. Of the 405 patients, 162 received surgery, and the rate of R0/1 resection reached 72%. The survival results showed that the 10-year overall survival (OS) was significantly higher in patients receiving surgery than in patients receiving other therapies (68.2% vs. 25.4%, P<0.001).
Extent of resection
Recommendation: For patients without myasthenia gravis, the goal of surgery is complete excision of the lesion with total thymectomy and complete resection of invaded tissues (1B). For patients with myasthenia gravis, further extended thymectomy (total thymectomy with resection of adjacent bilateral mediastinal pleura and fatty tissue within the mediastinum, peri-pericardium areas, and aortopulmonary window) is recommended (1C).
Total thymectomy via median sternotomy is the conventional surgical procedure for patients with thymic tumors. Removing the thymus in patients with early-stage tumors that can be completely resected is not technically difficult. In addition, the thymus loses its immune function in adults, so thymectomy theoretically will not cause a functional loss in these patients.
With the development of minimally invasive techniques, the opinion of total thymectomy has been challenged. According to a study of 1,047 patients with Masaoka-Koga stage I/II TETs based on the database of Chinese Alliance of Research for Thymomas (ChART) (22), nearly 1/4 of patients received partial thymectomy. In contrast, almost all patients with sternotomy received total thymectomy. However, the proportions of total and partial thymectomy were comparable in patients receiving minimally invasive surgery. Multivariate analysis showed that the 10-year OS was similar between patients who underwent total and partial thymectomy (90.9% vs. 89.4%, P=0.732). Although the recurrence rates after partial and total thymectomy were similar (3.2% vs. 1.4%, P=0.259) among patients with Masaoka-Koga stage I tumors, there were significantly more recurrent events in patients receiving partial thymectomy than in those with total thymectomy in patients with Masaok-Koga stage II diseases (14.5% vs. 2.9%, P=0.001). Given that it is difficult to discriminate Makaoka-Koga stage I (encapsulated) and stage II (microscopic infiltration of capsule or mediastinal fatty tissue) tumors through preoperative imaging or intraoperative exploration, as well as the possibility of multiple lesions within the mediastinum, total thymectomy, either via an open or minimally invasive procedure, is recommended to ensure the efficacy of surgical resection based on the anatomic resection principle as well as oncological principles.
Surgical approach
Recommendation (1A): On the premise of following oncological principles and ensuring the safety of operation, surgeons can choose conventional median sternotomy or minimally invasive surgery depending on different situations. Minimally invasive surgery is recommended for early-stage (UICC stage I or Masaoka-Koga stage I–II) tumors. In large clinical centers with mature techniques, minimally invasive surgery could be tried on UICC stage II–IIIa tumors as long as surgical and oncological principles are guaranteed.
The conventional approach is median sternotomy, which allows exposure of the mediastinum and both thoracic cavities and evaluation of gross capsular invasion, infiltration of the thymus and mediastinum fatty tissue, peri-tumor pleural adhesion, and involvement of surrounding structures.
Minimally invasive surgery is mainly applied to early-stage tumors. Gu et al. analyzed 1,087 patients of UICC stage I (Masaoka stage I/II) TETs in the ChART database (23). The results showed that after the median follow-up of 26 months for the video-assisted thoracoscopic surgery (VATS) group and 36 months for the open group, there was no significant difference in 5-year OS (85.7% vs. 93.1%, P=0.539), disease-free survival (DFS) (92.5% vs. 91.9%, P=0.773), cumulative incidence of recurrence (CIR) (7.1% vs. 5.8%, P=0.522), or the improvement rate of myasthenia gravis between the two groups (83.3% vs. 88.2%, P=0.589). This suggests that minimally invasive surgery can achieve similar long-term effects to open surgery. Another retrospective study in Japan compared the oncologic outcomes of VATS with those of sternotomy in 2,835 patients with TMs (24). The 5-year OS in VATS-treated patients reached 97.9%, similar to that in sternotomy-treated patients (P=0.74).
Technically, it is not difficult to resect a UICC stage II–IIIa tumor with limited invasion of the pericardium or adjacent lung tissues and achieve comparable completeness of resection via minimally invasive surgery. Gu et al. reported the perioperative and survival results of MIT (minimal invasive thymectomy) compared to MST (median sternotomy thymectomy) in patients with UICC stage T2–3 TETs (25). After propensity score matching, the MIT group had considerably less blood loss (P<0.001), fewer postoperative complications (P=0.048), a shorter duration of chest drainage (P<0.001), and a shorter hospitalization duration (P<0.001) than the MST group. The 5-year freedom from recurrence rate was comparable between the two groups (78.2% vs. 78.5%, P=0.942).
With the development of minimally invasive techniques, it is possible to obtain complete resection for patients with recurrent or metastatic tumors and those with previously advanced tumors but downstaged after induction therapy, apart from patients with UICC stage IIIa tumors with limited invasion. For patients who probably need multimodality treatment, minimally invasive surgery is superior in less surgical trauma and faster functional recovery so that patients can better tolerate adjuvant therapy to achieve desired oncological outcomes.
There is still no consensus on the specific diameter of TETs suitable for minimally invasive surgery. Previously, a tumor over 5 cm was considered a “large” tumor in most studies, and it is safe and feasible to perform minimally invasive surgery for TETs ≤5 cm (26). However, with the improvement of surgical techniques, it has been reported that the main reason for conversion in minimally invasive surgery for tumors over 5 cm is the invasion of great vessels. In addition, for tumors over 5 cm, minimally invasive surgery could achieve oncological results similar to those of open surgery (24). Therefore, compared to the extent of tumor invasion, tumor size is not a major factor affecting the choice of surgical approach. It should be noted that due to the limited space in the mediastinum, it is more difficult to perform minimally invasive surgery when the tumor becomes large. There would also be increased risks of pleural dissemination in this situation. Surgeons should strictly adhere to oncological principles during surgery. Conversion to open surgery should be carried out if complete resection is difficult or there is a risk of tumor spillage.
In addition, the surgical approach is not affected by the histological type, which is difficult to confirm before surgery for most early-stage tumors. Although the retrospective studies of ITMIG (27) and JART (28) did not include TCs, the study of the ChART (29) database showed that early-stage TC was not a contraindication of minimally invasive surgery as long as complete resection could be obtained.
Lymph node dissection
Recommendation (1B): Dissection of anterior mediastinal (N1) lymph nodes is recommended as a routine procedure, as N1 nodes are within the scope of total thymectomy. For tumors staged in T3 and above or those with high-grade histology (highly suspected or biopsy confirmed type B3 TM, TC, and NETT), further sampling of at least ipsilateral N2 lymph nodes is recommended. Bilateral N2 lymph node dissection is unnecessary, except for highly suspected NETTs with notable bilateral N2 nodes enlargement.
Lymph node metastasis is believed to be rare in TETs; thus, lymph node dissection is rarely carried out in conventional surgeries.
In recent years, the issue of lymph node metastasis has gained increasing attention. In the widely used Masaoka-Koga staging system, lymph node metastasis is grouped into stage IVb, together with distant metastasis. However, in the 8th edition of UICC staging, the node (N) category is divided into three tiers (N0–2) depending on the presence of nodal involvement in different anatomical regions. Recent studies have shown that the incidence of lymph node metastasis varies depending on the histological type and the extent of tumor invasion. A study of 1,320 patients from the JART database (30) found that the overall incidence of lymph node metastasis was only 5.9%. There were 1.8% of TMs, 27% of TCs, and 28% of thymic carcinoids accompanied by lymph node metastasis. Two studies based on the SEER database (31,32) included patients with surgical removal of at least one lymph node. The results showed that the incidence of lymph node metastasis was 13.3% in TMs, 33.5% in TCs, and 62.3% in NETTs.
According to the results of a retrospective study from the ChART database (33), among 2,421 patients in 20 hospitals, lymph node metastasis was rarely seen in TMs (only 0.5%) but occurred in 7.9% of TCs and 16.7% of NETTs. Moreover, lymph node metastasis was closely related to the prognosis of TETs.
Further prospective observational study from ChART (34) showed that lymph node involvement in thymic malignancies is more common than previously recognized. Through intentional lymph node sampling or systemic dissection, lymph node metastasis was seen in 2.1% of patients with TMs, 25% of TCs, and 50% of NETTs. N2 node dissection, along with higher-grade tumor histology and advanced T category, were found to be associated with increased nodal-positive rate. Thus, TETs were further divided into a low-risk group (type A-B2 TMs staged in T1–2) and a high-risk group with higher-grade histology (type B3 TM, TC, and NETT) or stage T3 and above for nodal metastasis. Intentional lymph node dissection could increase the detection rate of nodal involvement and improve the accuracy of staging and the completeness of resection. Theoretically, when total thymectomy is performed as recommended above, N1 nodes, located in the anterior mediastinum, would have already been dissected together. Considering that N2 involvement was usually on the ipsilateral side of tumor extension according to the ChART study, as well as that bilateral N2 node dissection would not be feasible via minimally invasive surgery with a unilateral approach, it is recommended that ipsilateral N2 nodes should at least be sampled for patients in the high-risk group.
Specimen handling
After resection, complete retrieval of the specimen should be done carefully to prevent specimen disruption. For minimally invasive surgery, the specimen should always be extracted in a retrieving bag (35). It is encouraged to handle the resected specimens as follows (36).
- Orienting the unfurled specimen on a mediastinal board or diagram is encouraged.
- Mark co-resected structures (mediastinal pleura, lung tissue, pericardium, phrenic nerve, blood vessels, etc.).
- Mark areas of concern, for example, margins of resection.
- Record the location of lymph nodes removed during operation.
- Provide the patient’s medical history, especially previous therapies and comorbidities, on the pathological examination application form and communicate with the pathologist promptly.
Adjuvant therapy
TM
Recommendation (1C): After complete resection, adjuvant therapy is not recommended for TMs in UICC stage I and type A/AB/B1 TMs in stage II–IIIa. Adjuvant radiotherapy or follow-up is alternative for type B2/3 TMs in UICC stage II–IIIa. Adjuvant radiotherapy is recommended for TMs with incomplete (R1/2) resection. Adjuvant chemotherapy is recommended for tumors with lymph node metastasis (Figure 4, Table 1).

A retrospective analysis based on the ChART database (37) involving 1,546 patients with Masaoka-Koga stage I–III TETs showed that adjuvant radiotherapy could improve OS and DFS for patients with R1/2 resection.
A retrospective analysis using the ITMIG data (38) from 1,263 patients with completely resected Masaoka stage II–III TMs showed that the 10-year OS was higher in patients with postoperative radiotherapy than those without (86% vs. 79%, P=0.002). Furthermore, for stage III type B TMs, postoperative radiotherapy could also significantly improve OS. However, the results from the JART database (39) showed that postoperative radiotherapy might be beneficial only in stage III TCs in terms of recurrence-free survival (RFS) but not OS. It could not improve RFS or OS for patients with Masaoka stage II TETs or stage II TCs.
In the NCCN Guidelines (2021. V1) (40), adjuvant radiotherapy is not recommended for completely resected (R0) Masaoka-Koga stage I TMs. Adjuvant radiotherapy can be considered for TMs with capsular invasion after an R0 resection. Postoperative radiotherapy is recommended for Masaoka-Koga stage III (invasion of adjacent structures) TMs because of the higher risk of recurrence.
However, depending on the recurrence predictive model based on the ChART database (41), patients with stage T1 TMs or stage T2–3 type A, AB, and B1 TMs (low-risk group) had a significantly lower incidence of recurrence than those with stage T2–3 type B2, B3 TMs and all TCs and NETTs (high-risk group) (2.7% vs. 20.1%, P<0.001).
Based on the above literature, adjuvant therapy is not recommended for patients with UICC stage I TMs and stage II–IIIa type A/AB/B1 TMs after an R0 resection. Adjuvant radiotherapy or follow-up can be considered in patients with UICC stage II–III A type B2/3 TMs.
For adjuvant chemotherapy, a retrospective study based on the ChART database included 739 patients with Masaoka-Koga stage III/IV TETs (42). Among patients with stage IV TMs, there was no difference in 5-year OS for patients with or without adjuvant chemotherapy (76.1% vs. 85.7%, P=0.862). However, for patients with stage III TMs, patients with adjuvant chemotherapy had worse 5-year OS than those without (88.1% vs. 92.1%, P<0.001). Moreover, among patients with completely resected TMs, the 5-year OS was significantly lower for patients with chemotherapy than those without (67.2% vs. 92.8%, P=0.001). Therefore, adjuvant chemotherapy is not recommended for patients with TMs. However, systemic therapy is still recommended for patients with lymph node metastasis (although lymph node metastasis is rare in TMs).
TC and NETT
Recommendation (1C): For completely resected TCs and NETTs, adjuvant chemotherapy with or without radiotherapy is recommended. For TCs and NETTs with an incomplete (R1/2) resection, adjuvant chemoradiotherapy is recommended (Figure 4, Table 1).
A retrospective study from Shanghai Chest Hospital included 116 patients with completely resected TCs (43). The results showed that adjuvant chemotherapy significantly improved the 5-year RFS for patients with Masaoka stage II tumors (84% vs. 66.6%, P=0.035) and the 5-year OS for patients with stage III tumors (84.6% vs. 63.7%, P=0.036).
A retrospective study based on the JART database included 1,265 patients with Masaoka stage II–III TETs (39). Data showed that postoperative radiotherapy was associated with better RFS [hazard ratio (HR) 0.48, 95% confidence interval (CI): 0.30–0.78, P=0.003] but not OS for stage III TC patients. Another meta-analysis of 592 patients of completely resected Masaoka stage II–III TETs showed that postoperative radiotherapy could not reduce the recurrence rate (44).
According to the recurrence predictive model proposed by ChART (41), patients with TCs and NETTs had a higher risk of recurrence, especially distant metastasis. Therefore, adjuvant chemotherapy with or without radiotherapy is recommended for completely resected TCs and NETTs.
Treatment for advanced tumors
Neoadjuvant therapy
Recommendation (1B): For locally advanced TETs, neoadjuvant chemotherapy or chemoradiotherapy is recommended, followed by a re-evaluation of surgical indications. Postoperative radiotherapy or chemoradiotherapy is recommended depending on the resection margins and pathological assessment. Definitive radiotherapy or chemoradiotherapy is recommended if the tumor is deemed unresectable after induction therapy (Figure 3). First-line chemotherapy regimens for TMs, TCs, and NETTs are CAP or TC, TC, and PE regimens, respectively (Table 1).
Recommendation (2C): For patients with pleural dissemination or intrapulmonary metastases, induction followed by surgery is an option.
Induction therapy followed by surgery might be effective for potentially resectable TETs (45-51).
A recent cohort study showed a similar 5-year OS for patients receiving induction chemotherapy followed by surgery to those receiving surgery alone (77.4% vs. 76.7%, P=0.596) (46).
To date, there have been two phase II clinical trials (51,52) studying the efficacy of induction chemotherapy. The objective response rate (ORR) reached 62% and 77%, respectively, with a high incidence of adverse events. The rate of pathological complete response (PCR) was 14% and 9%, and the rate of R0 resection was 43% and 73%, respectively. However, considering that both studies only accrued TMs and had a high proportion of low-grade subtypes, the actual efficacy of induction chemotherapy for high-grade TETs, especially for TCs, is unclear. According to the results of clinical trials in non-surgical patients, TCs respond poorly to chemotherapy (53,54).
According to a phase II clinical trial of neoadjuvant concurrent chemoradiotherapy for locally advanced high-grade TETs (type B2/B3 TM, TC, NETT) at Shanghai Chest Hospital, the ORR was 48.5% with tolerated toxicities. The rate of R0 resection was 82.6% for patients receiving surgery, and the rate of PCR reached 17.4%. The 5-year OS rates for TM and TC patients were 81.8% and 54.2%, respectively.
A previous phase II trial (55) from North America and Europe reported a 47.6% response rate and a 71% 5-year OS for locally advanced TETs after neoadjuvant concurrent chemoradiation followed by surgery.
Systemic therapy
Recommendation: Definitive chemoradiotherapy is recommended for unresectable advanced TETs (Figure 3) (1B). Surgery remains an option for patients with recurrent locally advanced lesions, solitary metastasis, or ipsilateral pleural metastasis (2C).
TM
Given different metastatic scenarios, it is sometimes difficult to specify radiation doses for metastatic lesions. Stereotactic body radiation therapy (SBRT) is an appropriate option for focal metastases, while conventional fractionation is suitable for larger metastatic lesions. In palliative treatment, typical palliative doses of 8 Gy/fx, 20 Gy/5 fxs or 30 Gy/10 fxs can be used, depending on the target. Even for metastatic TMs, due to their slow growth, highly conformal techniques might be suitable for lesions of limited size. It could be helpful to improve local control by increasing radiation doses. On the other hand, multiple radiotherapies for recurring metastatic lesions might increase the risk of radiation-induced lung injury.
Currently, the recommended first-line chemotherapy regimens for TMs are platinum-based regimens (CAP or TC) (56-59). The response rates of the CAP regimen for TMs are approximately 44% (60). Non-anthracycline regimens [e.g., cisplatin/etoposide (± ifosfamide), carboplatin/paclitaxel] are alternative for patients who cannot tolerate more aggressive regimens.
Second-line regimens for TMs include pemetrexed, everolimus, paclitaxel, octreotide [long-acting release (LAR)] with or without prednisolone, gemcitabine with or without capecitabine, 5-FU, etoposide, and ifosfamide (59,61-69). However, these drugs have not been assessed in randomized phase III trials. For TMs, response rates of subsequent systemic therapy range from 15% to 39% (60). A study of pemetrexed in the treatment of TM patients (n=16) reported 2 patients with complete response (CR) and 5 patients with partial response (PR) (70). Based on clinical trial data, capecitabine might be an effective addition to a single gemcitabine regimen (61,68). Among 22 TM patients treated with gemcitabine/capecitabine, 3 patients achieved CR, and 5 achieved PR. Octreotide may be an option for TM patients with a positive octreotide scan or symptoms of carcinoid syndrome. Pembrolizumab is not recommended for TM patients due to concerns about severe immune-related events. It was reported that 71.4% of TM patients receiving pembrolizumab had grade 3 or higher immune-related adverse events (71). Sunitinib is not recommended due to the scarcity of c-Kit mutations in TMs (72,73).
TC
TCs respond poorly to chemotherapy. Carboplatin/paclitaxel (TC) regimen is recommended as the first-line therapy for its highest reported response rate in clinical trials for TCs (53,54,74-82). Clinical data suggest that CAP and cisplatin/adriamycin/vincristine/cyclophosphamide (ADOC) regimens are also effective but more toxic (60,80).
There are limited data about second-line therapy for TCs. Alternative options include sunitinib, pemetrexed, everolimus, paclitaxel, octreotide (LAR) with or without prednisone, gemcitabine with or without capecitabine, 5-FU, etoposide, ifosfamide, and pembrolizumab (7,70,83). Response rates range from 4% to 21%. Sunitinib may be effective in patients with c-Kit mutations, but such mutations are rare in TCs (<10%) (64,72,84-88). S-1 (oral fluorouracil) seems to be effective for patients with TCs (89,90).
Pembrolizumab might be an effective second-line therapy for TC patients. According to the two trials of immunotherapy for thymic tumors, 19.2% and 22.5% of patients with TCs responded after receiving pembrolizumab (71,91). The incidence of severe immune-related adverse events was 15.4% and 15%, respectively. Capecitabine/gemcitabine is also suitable for TCs in the second-line setting (61,68). Three out of 8 patients with TCs had PR after receiving the gemcitabine/capecitabine regimen. In addition, the response rate of immune checkpoint inhibitors (ICIs) in combination of chemotherapy was reported to be 44.4% for advanced TCs, higher than that of ICIs monotherapy (17.4%) (92). The combination of ICIs and anti-angiogenesis drugs also has been explored in TCs (93). However, the efficacy and safety of combined therapies are in need for further study.
NETT
Patients with NETTs, especially the more aggressive subtypes, are more likely to have local invasion, lymph node metastasis and distant metastasis. Although SSAs were reported to be effective for neuroendocrine tumors (94), only 2 and 4 patients received SSAs before and after surgery, respectively. Given the limited efficacy of conventional chemotherapy and radiotherapy in treating NETTs, other agents, such as SSAs, should be explored, as well as mammalian target of rapamycin (mTOR) inhibitors (95) and multitarget drugs targeting vascular endothelial growth factor receptors (96) that have been tried in other neuroendocrine tumors.
Rehabilitation
The postoperative management of TET patients was similar to that of other patients undergoing thoracic surgery. In recent years, with the development of enhanced recovery after surgery (ERAS), recovery after thoracic surgery has gradually gained increasing attention. As current clinical evidence only focuses on recovery after lung surgery, the Clinical Practice Guidelines for ERAS in China (2021 Edition) (97) proposed by the Chinese Medical Association and the Guidelines for Enhanced Recovery After Lung Surgery (98) proposed by the European Society of Thoracic Surgeons (ESTS) can be referred to for enhanced recovery after thymic surgery.
It should be noted that for patients with myasthenia gravis, clinicians should pay attention to worsening symptoms or even myasthenia crisis. If so, clinicians should adjust medication promptly, strengthen monitoring, and apply for a consultation with a neurologist when needed.
Follow-up
Recommendation (1B): For patients in the low-risk group (stage T1 TMs, stage T2/T3 type A/AB/B1 TMs), follow-up annually for 10 years is recommended. For patients in the high-risk group (stage T2/T3 type B2/B3 TMs, all TCs and NETTs), follow-up is recommended every six months for three years and then annually for another 3 years at least (Figure 4).
Liu et al. (41) analyzed 907 patients with completely resected TETs based on the ChART database. The results showed that the recurrence rate in patients with stage T1 TMs and T2/T3 type A/AB/B1 TMs (low-risk group) was significantly lower than that in patients with stage T2/T3 type B2/B3 TMs and T1-T3 TCs and NETTs (high-risk group) (2.7% vs. 20.1%, P<0.001). In the low-risk group, the majority of recurrences occurred in the tumor bed and pleural cavity (88.9%). In the high-risk group, there were more patients with distant metastasis (40.7%) and pleural dissemination (25.9%), which mostly (55.2%) occurred within 3 years after surgery. Only one case of recurrence in the high-risk group occurred over 6 years after surgery, but local recurrence still could be seen 10 years after surgery in the low-risk group.
In addition, for patients who receive adjuvant therapies or suffer from advanced tumors, the frequency of follow-up and type of examination should be adjusted accordingly.
TM patients have an increased risk of developing second malignancies (99-101). Since there is no consensus on screening, routine physical examination is still important.
Methodology
A multidisciplinary guideline development group was established among members of the CACA Mediastinal Tumor Committee. Systemic literature review and two rounds of questionnaires regarding key clinical issues were carried out. The grading of recommendations assessment, development and evaluation (GRADE) approach was used to rate the quality of evidence and the strength of recommendations (Table 2) (102). We present this article in accordance with the RIGHT reporting checklist (available at https://med.amegroups.com/article/view/10.21037/med-23-54/rc).
Table 2
| Categories | Definition |
|---|---|
| Levels of evidence | |
| High (A) | We are very confident that the true effect lies close to that of the estimate of the effect |
| Moderate (B) | We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different |
| Low (C) | Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect |
| Very low (D) | We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect |
| Grades of recommendation | |
| Level 1 (strong) | The panel is highly confident that the desirable consequences of an intervention outweigh its undesirable consequences. We believe that all or almost all informed people would make the recommended choice for or against an intervention |
| Level 2 (weak) | The panel is less confident that the desirable consequences of an intervention outweigh its undesirable consequences. We believe that most informed people would choose the recommended course of action, but a substantial number would not |
Used with permission from Elsevier. GRADE, grading of recommendations assessment, development and evaluation.
Conclusions
These CACA guidelines focus on the clinical differential diagnosis of anterior mediastinal lesions, management of asymptomatic small anterior mediastinal nodules, pathological classification and staging systems of TETs, as well as principles of surgery, neoadjuvant and adjuvant therapies, systemic therapies for advanced TETs, and follow-up strategies after surgical resection. Due to the rarity of TETs and limited high-level evidence, there are still many controversies remaining and future researches are encouraged to solve these questions.
Acknowledgments
The authors thank members of the Chinese Association of Mediastinal Tumor, China Anti-Cancer Association for their input on the present guidelines.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the RIGHT reporting checklist. Available at https://med.amegroups.com/article/view/10.21037/med-23-54/rc
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-23-54/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-23-54/coif). W.F. serves as the Editor-in-Chief of Mediastinum. He also received funding from the National Natural Science Foundation of China (grant 82072569). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Teckie S, Qi S, Chelius M, et al. Long-term outcome of 487 patients with early-stage extra-nodal marginal zone lymphoma. Ann Oncol 2017;28:1064-9. [Crossref] [PubMed]
- Ramos-Casals M, la Civita L, de Vita S, et al. Characterization of B cell lymphoma in patients with Sjögren's syndrome and hepatitis C virus infection. Arthritis Rheum 2007;57:161-70. [Crossref] [PubMed]
- Hsu CH, Chan JK, Yin CH, et al. Trends in the incidence of thymoma, thymic carcinoma, and thymic neuroendocrine tumor in the United States. PLoS One 2019;14:e0227197. [Crossref] [PubMed]
- Henschke CI, Lee IJ, Wu N, et al. CT screening for lung cancer: prevalence and incidence of mediastinal masses. Radiology 2006;239:586-90. [Crossref] [PubMed]
- Rampinelli C, Preda L, Maniglio M, et al. Extrapulmonary malignancies detected at lung cancer screening. Radiology 2011;261:293-9. [Crossref] [PubMed]
- Yoon SH, Choi SH, Kang CH, et al. Incidental Anterior Mediastinal Nodular Lesions on Chest CT in Asymptomatic Subjects. J Thorac Oncol 2018;13:359-66. [Crossref] [PubMed]
- Litvak AM, Woo K, Hayes S, et al. Clinical characteristics and outcomes for patients with thymic carcinoma: evaluation of Masaoka staging. J Thorac Oncol 2014;9:1810-5. [Crossref] [PubMed]
- Gaur P, Leary C, Yao JC. Thymic neuroendocrine tumors: a SEER database analysis of 160 patients. Ann Surg 2010;251:1117-21. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Neuroendocrine and Adrenal Tumors. 2022; Version 2.2022.
- Fang W, Xu N, Shen Y, et al. Management of incidentally detected small anterior mediastinal nodules: Which way to go? Lung Cancer 2022;168:30-5. [Crossref] [PubMed]
- Zhang X, Ji C, Gu Z, et al. Correlation between Serum Cytokeratin 19 Fragment and the Clinicopathological Features and Prognosis of Thymic Epithelial Tumors. Zhongguo Fei Ai Za Zhi 2018;21:519-25. [PubMed]
- Barth TF, Leithäuser F, Joos S, et al. Mediastinal (thymic) large B-cell lymphoma: where do we stand? Lancet Oncol 2002;3:229-34. [Crossref] [PubMed]
- Shen Y, Gu Z, Ye J, et al. CT staging and preoperative assessment of resectability for thymic epithelial tumors. J Thorac Dis 2016;8:646-55. [Crossref] [PubMed]
- Munden RF, Carter BW, Chiles C, et al. Managing Incidental Findings on Thoracic CT: Mediastinal and Cardiovascular Findings. A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol 2018;15:1087-96. [Crossref] [PubMed]
- Marx A, Chan JKC, Chalabreysse L, et al. The 2021 WHO Classification of Tumors of the Thymus and Mediastinum: What Is New in Thymic Epithelial, Germ Cell, and Mesenchymal Tumors? J Thorac Oncol 2022;17:200-13. [Crossref] [PubMed]
- Roden AC, Fang W, Jain D, et al. Thymic Epithelial Tumours Histopathology Reporting Guide. 3rd edition. Sydney: International Collaboration on Cancer Reporting; 2022.
- Detterbeck FC, Nicholson AG, Kondo K, et al. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. J Thorac Oncol 2011;6:S1710-6. [Crossref] [PubMed]
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S65-72. [Crossref] [PubMed]
- Detterbeck FC, Zeeshan A. Thymoma: current diagnosis and treatment. Chin Med J (Engl) 2013;126:2186-91. [Crossref] [PubMed]
- Ried M, Potzger T, Sziklavari Z, et al. Extended surgical resections of advanced thymoma Masaoka stages III and IVa facilitate outcome. Thorac Cardiovasc Surg 2014;62:161-8. [PubMed]
- Mizuno T, Okumura M, Asamura H, et al. Surgical management of recurrent thymic epithelial tumors: a retrospective analysis based on the Japanese nationwide database. J Thorac Oncol 2015;10:199-205. [Crossref] [PubMed]
- Gu Z, Fu J, Shen Y, et al. Thymectomy versus tumor resection for early-stage thymic malignancies: a Chinese Alliance for Research in Thymomas retrospective database analysis. J Thorac Dis 2016;8:680-6. [Crossref] [PubMed]
- Gu Z, Chen C, Wang Y, et al. Video-assisted thoracoscopic surgery versus open surgery for Stage I thymic epithelial tumours: a propensity score-matched study. Eur J Cardiothorac Surg 2018;54:1037-44. [Crossref] [PubMed]
- Agatsuma H, Yoshida K, Yoshino I, et al. Video-Assisted Thoracic Surgery Thymectomy Versus Sternotomy Thymectomy in Patients With Thymoma. Ann Thorac Surg 2017;104:1047-53. [Crossref] [PubMed]
- Gu Z, Hao X, Liu Y, et al. Minimally Invasive Thymectomy Could Be Attempted for Locally Advanced Thymic Malignancies: A Real-World Study With Propensity Score-Matched Analysis. J Thorac Oncol 2023;18:640-9. [Crossref] [PubMed]
- Tagawa T, Yamasaki N, Tsuchiya T, et al. Thoracoscopic versus transsternal resection for early stage thymoma: long-term outcomes. Surg Today 2014;44:2275-80. [Crossref] [PubMed]
- Burt BM, Yao X, Shrager J, et al. Determinants of Complete Resection of Thymoma by Minimally Invasive and Open Thymectomy: Analysis of an International Registry. J Thorac Oncol 2017;12:129-36. [Crossref] [PubMed]
- Hess NR, Sarkaria IS, Pennathur A, et al. Minimally invasive versus open thymectomy: a systematic review of surgical techniques, patient demographics, and perioperative outcomes. Ann Cardiothorac Surg 2016;5:1-9. [PubMed]
- Wang H, Gu Z, Ding J, et al. Perioperative outcomes and long-term survival in clinically early-stage thymic malignancies: video-assisted thoracoscopic thymectomy versus open approaches. J Thorac Dis 2016;8:673-9. [Crossref] [PubMed]
- Kondo K, Monden Y. Lymphogenous and hematogenous metastasis of thymic epithelial tumors. Ann Thorac Surg 2003;76:1859-64; discussion 1864-5. [Crossref] [PubMed]
- Weksler B, Holden A, Sullivan JL. Impact of Positive Nodal Metastases in Patients with Thymic Carcinoma and Thymic Neuroendocrine Tumors. J Thorac Oncol 2015;10:1642-7. [Crossref] [PubMed]
- Weksler B, Pennathur A, Sullivan JL, et al. Resection of thymoma should include nodal sampling. J Thorac Cardiovasc Surg 2015;149:737-42. [Crossref] [PubMed]
- Gu Z, Wei Y, Fu J, et al. Lymph node metastases in thymic malignancies: a Chinese Alliance for Research in Thymomas retrospective database analysis. Interact Cardiovasc Thorac Surg 2017;25:455-61. [Crossref] [PubMed]
- Fang W, Wang Y, Pang L, et al. Lymph node metastasis in thymic malignancies: A Chinese multicenter prospective observational study. J Thorac Cardiovasc Surg 2018;156:824-833.e1. [Crossref] [PubMed]
- Toker A, Sonett J, Zielinski M, et al. Standard terms, definitions, and policies for minimally invasive resection of thymoma. J Thorac Oncol 2011;6:S1739-42. [Crossref] [PubMed]
- Detterbeck FC, Moran C, Huang J, et al. Which way is up? Policies and procedures for surgeons and pathologists regarding resection specimens of thymic malignancy. J Thorac Oncol 2011;6:S1730-8. [Crossref] [PubMed]
- Liu Q, Gu Z, Yang F, et al. The role of postoperative radiotherapy for stage I/II/III thymic tumor-results of the ChART retrospective database. J Thorac Dis 2016;8:687-95. [Crossref] [PubMed]
- Rimner A, Yao X, Huang J, et al. Postoperative Radiation Therapy Is Associated with Longer Overall Survival in Completely Resected Stage II and III Thymoma-An Analysis of the International Thymic Malignancies Interest Group Retrospective Database. J Thorac Oncol 2016;11:1785-92. [Crossref] [PubMed]
- Omasa M, Date H, Sozu T, et al. Postoperative radiotherapy is effective for thymic carcinoma but not for thymoma in stage II and III thymic epithelial tumors: the Japanese Association for Research on the Thymus Database Study. Cancer 2015;121:1008-16. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. Thymomas and Thymic Carcinomas. 2021; Version 1.2021. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1469
- Liu H, Gu Z, Qiu B, et al. A Recurrence Predictive Model for Thymic Tumors and Its Implication for Postoperative Management: a Chinese Alliance for Research in Thymomas Database Study. J Thorac Oncol 2020;15:448-56. [Crossref] [PubMed]
- Ma K, Gu Z, Han Y, et al. Application of Postoperative Chemotherapy on Thymomas and Its Prognostic Effect. Zhongguo Fei Ai Za Zhi 2016;19:473-82. [PubMed]
- Gao L, Wang C, Liu M, et al. Adjuvant chemotherapy improves survival outcomes after complete resection of thymic squamous cell carcinoma: a retrospective study of 116 patients. Interact Cardiovasc Thorac Surg 2021;33:550-6. [Crossref] [PubMed]
- Korst RJ, Kansler AL, Christos PJ, et al. Adjuvant radiotherapy for thymic epithelial tumors: a systematic review and meta-analysis. Ann Thorac Surg 2009;87:1641-7. [Crossref] [PubMed]
- Okereke IC, Kesler KA, Freeman RK, et al. Thymic carcinoma: outcomes after surgical resection. Ann Thorac Surg 2012;93:1668-72; discussion 1672-3. [Crossref] [PubMed]
- Park S, Park IK, Kim YT, et al. Comparison of Neoadjuvant Chemotherapy Followed by Surgery to Upfront Surgery for Thymic Malignancy. Ann Thorac Surg 2019;107:355-62. [Crossref] [PubMed]
- Ruffini E, Guerrera F, Brunelli A, et al. Report from the European Society of Thoracic Surgeons prospective thymic database 2017: a powerful resource for a collaborative global effort to manage thymic tumours. Eur J Cardiothorac Surg 2019;55:601-9. [Crossref] [PubMed]
- Kanzaki R, Kanou T, Ose N, et al. Long-term outcomes of advanced thymoma in patients undergoing preoperative chemotherapy or chemoradiotherapy followed by surgery: a 20-year experience. Interact Cardiovasc Thorac Surg 2019;28:360-7. [Crossref] [PubMed]
- Riely GJ, Huang J. Induction therapy for locally advanced thymoma. J Thorac Oncol 2010;5:S323-6. [Crossref] [PubMed]
- Wright CD, Choi NC, Wain JC, et al. Induction chemoradiotherapy followed by resection for locally advanced Masaoka stage III and IVA thymic tumors. Ann Thorac Surg 2008;85:385-9. [Crossref] [PubMed]
- Kim ES, Putnam JB, Komaki R, et al. Phase II study of a multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation therapy, and consolidation chemotherapy for unresectable malignant thymomas: final report. Lung Cancer 2004;44:369-79. [Crossref] [PubMed]
- Kunitoh H, Tamura T, Shibata T, et al. A phase II trial of dose-dense chemotherapy, followed by surgical resection and/or thoracic radiotherapy, in locally advanced thymoma: report of a Japan Clinical Oncology Group trial (JCOG 9606). Br J Cancer 2010;103:6-11. [Crossref] [PubMed]
- Igawa S, Murakami H, Takahashi T, et al. Efficacy of chemotherapy with carboplatin and paclitaxel for unresectable thymic carcinoma. Lung Cancer 2010;67:194-7. [Crossref] [PubMed]
- Lemma GL, Lee JW, Aisner SC, et al. Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma. J Clin Oncol 2011;29:2060-5. [Crossref] [PubMed]
- Korst RJ, Bezjak A, Blackmon S, et al. Neoadjuvant chemoradiotherapy for locally advanced thymic tumors: a phase II, multi-institutional clinical trial. J Thorac Cardiovasc Surg 2014;147:36-44, 46.e1.
- Kondo K. Optimal therapy for thymoma. J Med Invest 2008;55:17-28. [Crossref] [PubMed]
- Okuma Y, Saito M, Hosomi Y, et al. Key components of chemotherapy for thymic malignancies: a systematic review and pooled analysis for anthracycline-, carboplatin- or cisplatin-based chemotherapy. J Cancer Res Clin Oncol 2015;141:323-31. [Crossref] [PubMed]
- Rajan A, Giaccone G. Chemotherapy for thymic tumors: induction, consolidation, palliation. Thorac Surg Clin 2011;21:107-14. viii. [Crossref] [PubMed]
- Schmitt J, Loehrer PJ Sr. The role of chemotherapy in advanced thymoma. J Thorac Oncol 2010;5:S357-60. [Crossref] [PubMed]
- Merveilleux du Vignaux C, Dansin E, Mhanna L, et al. Systemic Therapy in Advanced Thymic Epithelial Tumors: Insights from the RYTHMIC Prospective Cohort. J Thorac Oncol 2018;13:1762-70. [Crossref] [PubMed]
- Palmieri G, Buonerba C, Ottaviano M, et al. Capecitabine plus gemcitabine in thymic epithelial tumors: final analysis of a Phase II trial. Future Oncol 2014;10:2141-7. [Crossref] [PubMed]
- Bluthgen MV, Boutros C, Fayard F, et al. Activity and safety of oral etoposide in pretreated patients with metastatic or recurrent thymic epithelial tumors (TET): A single-institution experience. Lung Cancer 2016;99:111-6. [Crossref] [PubMed]
- Zucali PA, De Pas T, Palmieri G, et al. Phase II Study of Everolimus in Patients With Thymoma and Thymic Carcinoma Previously Treated With Cisplatin-Based Chemotherapy. J Clin Oncol 2018;36:342-9. [Crossref] [PubMed]
- Thomas A, Rajan A, Berman A, et al. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: an open-label phase 2 trial. Lancet Oncol 2015;16:177-86. [Crossref] [PubMed]
- Liang Y, Padda SK, Riess JW, et al. Pemetrexed in patients with thymic malignancies previously treated with chemotherapy. Lung Cancer 2015;87:34-8. [Crossref] [PubMed]
- Longo F, De Filippis L, Zivi A, et al. Efficacy and tolerability of long-acting octreotide in the treatment of thymic tumors: results of a pilot trial. Am J Clin Oncol 2012;35:105-9. [Crossref] [PubMed]
- Loehrer PJ Sr, Wang W, Johnson DH, et al. Octreotide alone or with prednisone in patients with advanced thymoma and thymic carcinoma: an Eastern Cooperative Oncology Group Phase II Trial. J Clin Oncol 2004;22:293-9. [Crossref] [PubMed]
- Palmieri G, Merola G, Federico P, et al. Preliminary results of phase II study of capecitabine and gemcitabine (CAP-GEM) in patients with metastatic pretreated thymic epithelial tumors (TETs). Ann Oncol 2010;21:1168-72. [Crossref] [PubMed]
- Highley MS, Underhill CR, Parnis FX, et al. Treatment of invasive thymoma with single-agent ifosfamide. J Clin Oncol 1999;17:2737-44. [Crossref] [PubMed]
- Gbolahan OB, Porter RF, Salter JT, et al. A Phase II Study of Pemetrexed in Patients with Recurrent Thymoma and Thymic Carcinoma. J Thorac Oncol 2018;13:1940-8. [Crossref] [PubMed]
- Cho J, Kim HS, Ku BM, et al. Pembrolizumab for Patients With Refractory or Relapsed Thymic Epithelial Tumor: An Open-Label Phase II Trial. J Clin Oncol 2019;37:2162-70. [Crossref] [PubMed]
- Ströbel P, Bargou R, Wolff A, et al. Sunitinib in metastatic thymic carcinomas: laboratory findings and initial clinical experience. Br J Cancer 2010;103:196-200. [Crossref] [PubMed]
- Dai J, Song N, Yang Y, et al. Is it valuable and safe to perform reoperation for recurrent thymoma? Interact Cardiovasc Thorac Surg 2015;21:526-31. [Crossref] [PubMed]
- Hirai F, Yamanaka T, Taguchi K, et al. A multicenter phase II study of carboplatin and paclitaxel for advanced thymic carcinoma: WJOG4207L. Ann Oncol 2015;26:363-8. [Crossref] [PubMed]
- Furugen M, Sekine I, Tsuta K, et al. Combination chemotherapy with carboplatin and paclitaxel for advanced thymic cancer. Jpn J Clin Oncol 2011;41:1013-6. [Crossref] [PubMed]
- Maruyama R, Suemitsu R, Okamoto T, et al. Persistent and aggressive treatment for thymic carcinoma. Results of a single-institute experience with 25 patients. Oncology 2006;70:325-9. [Crossref] [PubMed]
- Weide LG, Ulbright TM, Loehrer PJ Sr, et al. Thymic carcinoma. A distinct clinical entity responsive to chemotherapy. Cancer 1993;71:1219-23. [Crossref] [PubMed]
- Lucchi M, Mussi A, Ambrogi M, et al. Thymic carcinoma: a report of 13 cases. Eur J Surg Oncol 2001;27:636-40. [Crossref] [PubMed]
- Yoh K, Goto K, Ishii G, et al. Weekly chemotherapy with cisplatin, vincristine, doxorubicin, and etoposide is an effective treatment for advanced thymic carcinoma. Cancer 2003;98:926-31. [Crossref] [PubMed]
- Koizumi T, Takabayashi Y, Yamagishi S, et al. Chemotherapy for advanced thymic carcinoma: clinical response to cisplatin, doxorubicin, vincristine, and cyclophosphamide (ADOC chemotherapy). Am J Clin Oncol 2002;25:266-8. [Crossref] [PubMed]
- Kanda S, Koizumi T, Komatsu Y, et al. Second-line chemotherapy of platinum compound plus CPT-11 following ADOC chemotherapy in advanced thymic carcinoma: analysis of seven cases. Anticancer Res 2007;27:3005-8. [PubMed]
- Komatsu Y, Koizumi T, Tanabe T, et al. Salvage chemotherapy with carboplatin and paclitaxel for cisplatin-resistant thymic carcinoma--three cases. Anticancer Res 2006;26:4851-5. [PubMed]
- Girard N. Chemotherapy and targeted agents for thymic malignancies. Expert Rev Anticancer Ther 2012;12:685-95. [Crossref] [PubMed]
- Kelly RJ, Petrini I, Rajan A, et al. Thymic malignancies: from clinical management to targeted therapies. J Clin Oncol 2011;29:4820-7. [Crossref] [PubMed]
- Remon J, Girard N, Mazieres J, et al. Sunitinib in patients with advanced thymic malignancies: Cohort from the French RYTHMIC network. Lung Cancer 2016;97:99-104. [Crossref] [PubMed]
- Palmieri G, Marino M, Buonerba C, et al. Imatinib mesylate in thymic epithelial malignancies. Cancer Chemother Pharmacol 2012;69:309-15. [Crossref] [PubMed]
- Bisagni G, Rossi G, Cavazza A, et al. Long lasting response to the multikinase inhibitor bay 43-9006 (Sorafenib) in a heavily pretreated metastatic thymic carcinoma. J Thorac Oncol 2009;4:773-5. [Crossref] [PubMed]
- Ströbel P, Hartmann M, Jakob A, et al. Thymic carcinoma with overexpression of mutated KIT and the response to imatinib. N Engl J Med 2004;350:2625-6. [Crossref] [PubMed]
- Okuma Y, Shimokawa T, Takagi Y, et al. S-1 is an active anticancer agent for advanced thymic carcinoma. Lung Cancer 2010;70:357-63. [Crossref] [PubMed]
- Wang CL, Gao LT, Lu CX. S-1 salvage chemotherapy for stage IV thymic carcinoma: a study of 44 cases. J Thorac Dis 2019;11:2816-21. [Crossref] [PubMed]
- Giaccone G, Kim C, Thompson J, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol 2018;19:347-55. [Crossref] [PubMed]
- Wang W, Lin G, Hao Y, et al. Treatment outcomes and prognosis of immune checkpoint inhibitors therapy in patients with advanced thymic carcinoma: A multicentre retrospective study. Eur J Cancer 2022;174:21-30. [Crossref] [PubMed]
- Conforti F, Zucali PA, Pala L, et al. Avelumab plus axitinib in unresectable or metastatic type B3 thymomas and thymic carcinomas (CAVEATT): a single-arm, multicentre, phase 2 trial. Lancet Oncol 2022;23:1287-96. [Crossref] [PubMed]
- Kos-Kudła B. Treatment of neuroendocrine tumors: new recommendations based on the CLARINET study. Contemp Oncol (Pozn) 2015;19:345-9. [Crossref] [PubMed]
- Gajate P, Martínez-Sáez O, Alonso-Gordoa T, et al. Emerging use of everolimus in the treatment of neuroendocrine tumors. Cancer Manag Res 2017;9:215-24. [Crossref] [PubMed]
- Grande E, Capdevila J, Castellano D, et al. Pazopanib in pretreated advanced neuroendocrine tumors: a phase II, open-label trial of the Spanish Task Force Group for Neuroendocrine Tumors (GETNE). Ann Oncol 2015;26:1987-93. [Crossref] [PubMed]
- Li Y, Qiu J, Cao H. Application of enhanced recovery after surgery for patients with laparoscopic radical gastrectomy. Zhonghua Wei Chang Wai Ke Za Zhi 2016;19:269-73. [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol 2010;5:S260-5. [Crossref] [PubMed]
- Kumar V, Garg M, Goyal A, et al. Changing pattern of secondary cancers among patients with malignant thymoma in the USA. Future Oncol 2018;14:1943-51. [Crossref] [PubMed]
- Pan CC, Chen PC, Wang LS, et al. Thymoma is associated with an increased risk of second malignancy. Cancer 2001;92:2406-11. [Crossref] [PubMed]
- Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383-94. [Crossref] [PubMed]
Cite this article as: Fang W, Yu Z, Chen C, Chen G, Chen K, Fu J, Han Y, Fu X, Wang J, Mao T, Gu Z, Xu N. China Anti-Cancer Association Guidelines for the diagnosis, treatment, and follow-up of thymic epithelial tumors (2023). Mediastinum 2024;8:27.


