
Pathological features of thymoma and thymic carcinoma with therapeutic implications
Introduction
Primary R0 surgical resection is the key therapeutic aim to eradicate thymomas (TMs) and thymic carcinomas (TCs). If this is impossible, resection or debulking after neoadjuvant chemo-therapy or somatostatin/prednisone ‘induction’; postoperative radiotherapy (PORT); ‘definite chemotherapy’ in disseminated and recurrent, unresectable disease; and various “targeted” approaches alone or in combination with chemotherapy have been strategies to combat these cancers. Immune check-point inhibitors are currently in early phase clinical testing, revealing efficacy but also a high risk of dangerous autoimmune adverse effects, particularly in TM, i.e., the tumor with the highest frequency of paraneoplastic autoimmune phenomena (1). Since predictive biomarkers are largely missing (2), pathological features of TMs and TCs—(immuno-)histological properties, stage and resection status—continue to play the most relevant roles in guiding treatment decisions, as detailed next.
Pathological features with treatment implications
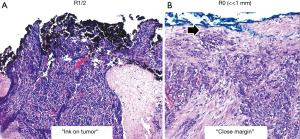
Histotype is usually the first feature recognized on needle biopsy of a mediastina mass of unknown differentiation [a clinical diagnosis of thymoma is almost certain only in patients with Myasthenia gravis, while positive tumor markers like AFP, HCG and neuroendocrine markers hint to subsets of germ cell tumors and neuroendocrine tumors, respectively, entailing therapeutic consequences very different from those of TMs and TCs (3)]. If a histological diagnosis of TM or TCs is made, imaging studies become key to make a decision either for primary resection or neoadjuvant approaches. While neoadjuvant chemotherapy is the standard approach in unresectable TMs and TCs alike (4), a histological diagnosis of lymphocyte-rich AB, B1 or B2 thymoma in conjunction with a positive ‘octreoscan’ may, alternatively, argue for a (chemotherapy-sparing) neoadjuvant octreotide/prednisone intervention to achieve secondary resectability (5). Histology may also affect the decision for PORT: there is some evidence that patients with type B2 and B3 TMs and TCs at low Masaoka-Koga stages (MKS) I or II but not patients with A or AB TMs profit from PORT after R0 resection (4). Also in the adjuvant setting, the distinction between B3 thymoma and TC appears to be important, since only TCs responded to the TKI, sunitinib in a recent phase II clinical trial (6). Immunohistochemistry (IHC) for NUT or neuroendocrine markers, respectively is key to distinguish NUT carcinomas and Large cell neuroendocrine carcinomas from poorly differentiated squamous cell carcinomas, small cell carcinomas or undifferentiated carcinomas, to draw better therapeutic conclusions (7). Whether quantifying PD-L1 expression by IHC in TMs and TCs is a valid predictive marker for therapies with immune checkpoint inhibitors is currently unclear (8,9). Tumor stage is one of the strongest independent prognostic features of TMs and TCs. MKS I/II TMs are usually cured by surgery alone and may not need adjuvant therapy (4), although recent studies suggest that PORT may also improve the control of some stage IIA tumors (10,11). In any case, advanced tumor stages irrespective of TM and TC histotype or resection status entail PORT (MKS III), pleurectomy with or without hyperthermic intrathoracic chemotherapy (HITOC) (stage IVa) (12) and/or chemotherapy (MKS IVb) (4). Resection status is not only an important prognostic factor (13,14), but the histological diagnosis of incomplete resection (R1 or R2)—defined as “ink on tumor”—typically is an indication for PORT irrespective of histotype, as PORT significantly improves survival in TMs and TCs (4,10,11). By contrast, the role of ‘close margins’ (distance of tumor to the surface of the given resection specimen) has not been evaluated in terms of recurrence and survival (Figure 1).
Conclusions
Taking into account that tumor stage, resection status and histotype are currently the most important factors in terms of prognosis, histopathological tumor features remain the basis of therapeutic decisions in almost all TMs and TCs. Molecular markers hopefully will play an increasingly important role in the futures, as exemplified by the rare TCs with KIT and NUT mutations (7,15).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors Mirella Marino and Brett W. Carter for the series “Dedicated to the 8th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2017)” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2017.12.03). The series “Dedicated to the 8th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2017)” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Marx A, Willcox N, Leite MI, et al. Thymoma and paraneoplastic myasthenia gravis. Autoimmunity 2010;43:413-27. [Crossref] [PubMed]
- Marx A, Weis CA. Sunitinib in thymic carcinoma: enigmas still unresolved. Lancet Oncol 2015;16:124-5. [Crossref] [PubMed]
- Girard N. Neuroendocrine tumors of the thymus: the oncologist point of view. J Thorac Dis 2017;9:S1491-500. [Crossref] [PubMed]
- Girard N, Ruffini E, Marx A, et al. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v40-55. [Crossref] [PubMed]
- Kirzinger L, Boy S, Marienhagen J, et al. Octreotide LAR and Prednisone as Neoadjuvant Treatment in Patients with Primary or Locally Recurrent Unresectable Thymic Tumors: A Phase II Study. PLoS One 2016;11:e0168215 [Crossref] [PubMed]
- Thomas A, Rajan A, Berman A, et al. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: an open-label phase 2 trial. Lancet Oncol 2015;16:177-86. [Crossref] [PubMed]
- Bragelmann J, Dammert MA, Dietlein F, et al. Systematic Kinase Inhibitor Profiling Identifies CDK9 as a Synthetic Lethal Target in NUT Midline Carcinoma. Cell Rep 2017;20:2833-45. [Crossref] [PubMed]
- Giaccone G, Thompson J, McGuire C, et al. Pembrolizumab in patients with recurrent thymic carcinoma: Results of a phase II study. J Clin Oncol 2017;35:8573.
- Rajan A, Heery CR, Perry S, et al. Safety and clinical activity of anti-programmed death-ligand 1 (PD-L1) antibody (ab) avelumab (MSB0010718C) in advanced thymic epithelial tumors (TETs). J Clin Oncol 2016;34:e20106
- Jackson MW, Palma DA, Camidge DR, et al. The Impact of Postoperative Radiotherapy for Thymoma and Thymic Carcinoma. J Thorac Oncol 2017;12:734-44. [Crossref] [PubMed]
- Rimner A, Yao X, Huang J, et al. Postoperative Radiation Therapy Is Associated with Longer Overall Survival in Completely Resected Stage II and III Thymoma-An Analysis of the International Thymic Malignancies Interest Group Retrospective Database. J Thorac Oncol 2016;11:1785-92. [Crossref] [PubMed]
- Ried M, Marx A, Gotz A, et al. State of the art: diagnostic tools and innovative therapies for treatment of advanced thymoma and thymic carcinoma. Eur J Cardiothorac Surg 2016;49:1545-52. [Crossref] [PubMed]
- Lococo F, Perotti G, Cardillo G, et al. Multicenter comparison of 18F-FDG and 68Ga-DOTA-peptide PET/CT for pulmonary carcinoid. Clin Nucl Med 2015;40:e183-9. [Crossref] [PubMed]
- Weis CA, Yao X, Deng Y, et al. The impact of thymoma histotype on prognosis in a worldwide database. J Thorac Oncol 2015;10:367-72. [Crossref] [PubMed]
- Strobel P, Hartmann M, Jakob A, et al. Thymic carcinoma with overexpression of mutated KIT and the response to imatinib. N Engl J Med 2004;350:2625-6. [Crossref] [PubMed]
Cite this article as: Marx A, Ströbel P, Schalke B, Weis CA. Pathological features of thymoma and thymic carcinoma with therapeutic implications. Mediastinum 2018;2:1.


